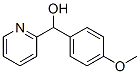
4-methoxy-alpha-pyridylbenzyl alcohol synthesis
- Product Name:4-methoxy-alpha-pyridylbenzyl alcohol
- CAS Number:27805-39-6
- Molecular formula:C13H13NO2
- Molecular Weight:215.25
1121-60-4
511 suppliers
$10.60/10gm:
699-19-4
0 suppliers
inquiry

27805-39-6
15 suppliers
$32.29/250mg
Yield: 82%
Reaction Conditions:
in tetrahydrofuran at -78 - 20; for 0.25 h;
Steps:
(4-Methoxyphenyl)(pyridin-2-yl)methanol (8i)
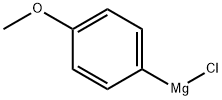
To a solution of picolinaldehyde (1.08 g, 10.1 mmol) in THF (50 mL) was added dropwise 4-methoxyphenylmagnesium chloride (20 mL, 10 mmol, 0.5 M in THF) at -78 °C. The reaction mixture was stirred at -78 °C for 5 min, and then room temperature for 10 min. The reaction was quenched by the addition of saturated aqueous ammonium chloride, and then extracted with ethyl acetate. The conbined organic layer was washed with brine, and dried over Na2SO4. The mixture was concentrated in vacuo. The crude material was purified by flash column chromatography on silica gel (Hexane:AcOEt = 1:1) to give 8i as a colorless solid (1.77 g, 82%). 1H NMR spectroscopic data are identical to those reported in the literature.2 Mp 130-132 °C. 1H NMR (400 MHz, CDCl3) δ 3.78 (3H, s), 5.21 (1H, d, J = 3.7 Hz), 5.71 (1H, d, J = 3.7 Hz), 6.87 (2H, d, J = 8.5 Hz), 7.13 (1H, d, J = 7.3 Hz), 7.18 (1H, dd, J = 7.3, 5.5 Hz), 7.28 (2H, d, J = 8.5 Hz), 7.61 (1H, ddd, J = 7.3, 7.3, 1.8 Hz), 8.56 (1H, d, J = 5.5 Hz). 13C NMR (100 MHz, CDCl3) δ 55.2, 74.5, 113.9, 121.3, 122.3, 128.4, 135.5, 136.8, 147.7, 159.2, 161.2. IR (ATR) nmax 3173, 1607, 1591, 1569, 1515, 1466, 1436, 1333, 1304, 1255, 1178, 1115, 1052, 1031, 1000 cm-1. MS (ESI) 216 (M+H)+. HRMS (ESI) calcd for C13H14NO2 (M+H)+ 216.10245, found 216.10212.
References:
Nishigaya, Yosuke;Umei, Kentaro;Watanabe, Daisuke;Kohno, Yasushi;Seto, Shigeki [Tetrahedron,2016,vol. 72,# 12,p. 1566 - 1572] Location in patent:supporting information
109-04-6
528 suppliers
$6.00/25g
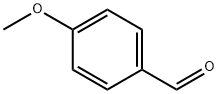
123-11-5
863 suppliers
$10.00/10g

27805-39-6
15 suppliers
$32.29/250mg

1121-60-4
511 suppliers
$10.60/10gm:
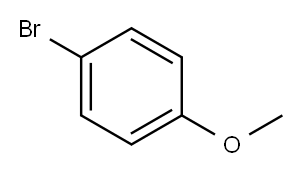
104-92-7
427 suppliers
$10.00/10g

27805-39-6
15 suppliers
$32.29/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

27805-39-6
15 suppliers
$32.29/250mg

1121-60-4
511 suppliers
$10.60/10gm:

13139-86-1
131 suppliers
$45.00/5g

27805-39-6
15 suppliers
$32.29/250mg