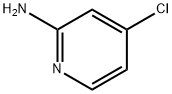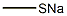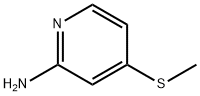
4-(Methylthio)pyridin-2-aMine synthesis
- Product Name:4-(Methylthio)pyridin-2-aMine
- CAS Number:38240-26-5
- Molecular formula:C6H8N2S
- Molecular Weight:140.21
Yield:38240-26-5 85%
Reaction Conditions:
in ethanol;water at 140; for 18 h;Sealed tube;
Steps:
43.1 Step 1:
A mixture of 4-chloro-2-aminopyridine (3.00 g, 23.3 mmol) andNaSMe (4.91 g, 70 mmol) in EtOH (37 mL) and EhO (9 mL) was stirred at 140°C for 18 h in a sealed tube. The mixture was diluted with water (150 mL) and adjusted to pH = 3 with aq. HC1 (2 M). The mixture was extracted with DCM (30 mL) and the organic layer discarded. The aqueous layer was adjusted to pH = 8 with sat. NaHCCh. The mixture was extracted with DCM (50 mL x 3). The organic layer was dried over NaiSCh, filtered and concentrated and the residue triturated with 5:1 PE/EA (20 mL) to reveal 4-(methylthio)-2-aminopyridine (2.8 g, 85%). LCMS (ESI, m/z): 141.3 [M+H]+. NMR (400MHz, CDCh) 7.88 (d, J = 5.5 Hz, 1H), 6.50 (br d, J = 4.5 Hz, 1H), 6.29 (s, 1H), 4.43 (br s, 2H), 2.43 (s, 3H).
References:
WO2021/161105,2021,A1 Location in patent:Paragraph 00345