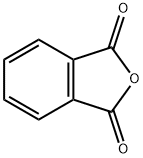
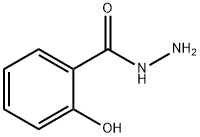
N-[2-(1,3-Dihudro-1,3-dioxo-2H-isoindolys)]2-hydroxybenzoylamide synthesis
- Product Name:N-[2-(1,3-Dihudro-1,3-dioxo-2H-isoindolys)]2-hydroxybenzoylamide
- CAS Number:57187-42-5
- Molecular formula:C15H10N2O4
- Molecular Weight:282.25
Yield:57187-42-5 85%
Reaction Conditions:
with acetic acid at 130; for 3 h;Reflux;
Steps:
1.3
1.3. Synthesis of compound N (1,3-dioxy-1,3-dihydro-2H-isoindol-2-yl) - 2-hydroxybenzamide In a 50 mL flask connected to a reflux condenser, add 0.5 g (3.28 mmol) of salicylic acid hydrazide obtained in stage 1.2, 15 mL of glacial acetic acid and 0.487 g of phthalic anhydride. The reaction is kept under reflux and stirring (130° C) for 3 hours until we can observe the formation of the product by thin layer chromatography (Mobile phase: 90% dichloromethane; 10% methanol). The reaction is cooled in an ice bath, with the formation of precipitate which is filtered and washed with cold water to provide 787 mg of a white solid (C15H10N2O4, MW=282.06, melting point: 241°-244°C; yield: 85%). The NMR H1 spectrum (400MHz, DMSOd) is as follows: δ 6.99-7.08 (2H); 7.51 (ddd; 1H; Jorto=8.36 Hz and Jmeta=1.71 Hz ) 7.94 (dd; 1H; Jorto=7.85 Hz and Jmeta=1.71 Hz); 7.96 (m; 2H; Jorto=8.7 Hz and Jmeta=2.73 Hz); 8.02 (m; 2H; Jorto=8.7 Hz and Jmeta=2.73 Hz); 11.10 (1H) ppm. The NMR C13 spectrum (400MHz, DMSOd) is as follows: δ 166.35; 165.47; 158.15; 135.6; 134.94; 130.14; 129.73; 124.03; 119.61; 117.46; 115.3 ppm.
References:
EP2418200,2012,A1 Location in patent:Page/Page column 28-29
119-36-8
989 suppliers
$5.00/10g
![N-[2-(1,3-Dihudro-1,3-dioxo-2H-isoindolys)]2-hydroxybenzoylamide](/CAS/GIF/57187-42-5.gif)
57187-42-5
12 suppliers
inquiry
69-72-7
1224 suppliers
$5.00/10g
![N-[2-(1,3-Dihudro-1,3-dioxo-2H-isoindolys)]2-hydroxybenzoylamide](/CAS/GIF/57187-42-5.gif)
57187-42-5
12 suppliers
inquiry