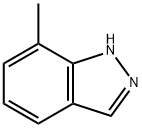
7-METHYL (1H)INDAZOLE synthesis
- Product Name:7-METHYL (1H)INDAZOLE
- CAS Number:3176-66-7
- Molecular formula:C8H8N2
- Molecular Weight:132.16
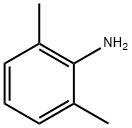
87-62-7
456 suppliers
$10.00/5 g

3176-66-7
121 suppliers
$50.00/1g
Yield: 85%
Reaction Conditions:
Stage #1:2,6-dimethylaniline with tert.-butylnitrite in chloroform at 20; for 0.333333 h;
Stage #2: with 18-crown-6 ether;potassium acetate in chloroform at 20; for 18 h;Heating / reflux;
Steps:
33.A
Example 33; 3-{2-Methyl-4-[1-(4-trifluoromethylphenyl)-1H-indazol-7- ylmethylsulfanyllphenvHpropionic Acid; Step A; 7-Methyl-lH-indazole; Add tert-butyl nitrite (52 mL) to a solution of commercially available 2,6- dimethylaniline (25 g, 206 mmol) in chloroform (750 mL) at room temperature under nitrogen, stir the mixture for 20 min and add potassium acetate (40 g, 412 mmol) and 18- crown-6 (5.4 g, 20.6 mmol). Heat the mixture at reflux for 3 h, cool to room temperature and stir for an additional 15 h. Remove the solids from the cooled mixture by vacuum filtration and wash with chloroform (400 mL). Wash the filtrate with water (2 x 250 mL), dry over MgS04 and remove the solvents were removed under reduced pressure. Purify the residue by flash column chromatography on silica gel, eluting with hexanes/ethyl acetate (9: 1), to afford 7-methyl-lH-indazole as an orange solid (23 g, 85%): IH NMR (CDC13) 8 2.60 (s, 3H), 7.10 (m, 2H), 7.70 (d, 1H), 8. 10 (s, 1H).
References:
Location in patent:Page/Page column 108-109

824-42-0
162 suppliers
$11.00/1g

3176-66-7
121 suppliers
$50.00/1g
2192-33-8
1 suppliers
inquiry

3176-66-7
121 suppliers
$50.00/1g
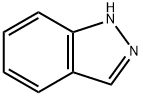
271-44-3
246 suppliers
$6.00/250mg

74-88-4
345 suppliers
$15.00/10g

3176-66-7
121 suppliers
$50.00/1g
679795-02-9
0 suppliers
inquiry

3176-66-7
121 suppliers
$50.00/1g