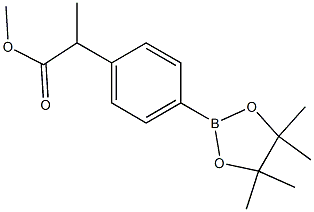
methyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate synthesis
- Product Name:methyl 2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)propanoate
- CAS Number:890839-11-9
- Molecular formula:C16H23BO4
- Molecular Weight:290.16
Yield:890839-11-9 92%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane; for 6 h;Inert atmosphere;Reflux;
Steps:
4.28. Methyl 2-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)phenyl]propionate (44)
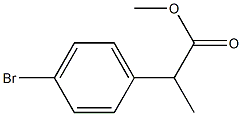
A mixture of Methyl 2-(4-bromophenyl)propionate (38) (5g, 20.56 mmol), potassium acetate (4.04 g, 41.11 mmol), Bis(pinacolato)diboron (10.45 g, 41.13 mmol) and [1,10-bis(diphenylphosphino)ferrocene]dichloropalladium (II) (0.84 g, 1.03 mmol) in 1,4-dioxane (100 mL) was refluxed for 6 h under nitrogen atmosphere. The reaction mixture was cooled to 60 °C and concentrated under reduced pressure. Water (50 mL) and ethyl acetate (50 mL) were charged, stirred for 30 min and filtered through celite. Organic layer was separated, dried over sodium sulfate and concentrated. The residue was purified using column chromatography (silica gel, 3-5 % ethyl acetate in hexane) to afford Methyl 2-[4-(4,4,5,5-tetramethyl-[1,3,2]dioxaboralan-2-yl)phenyl]propionate (44) (5.5 g, 92 %) as a colourless syrup: 1H NMR (400 MHz, CDCl3) δ 7.78 (d, J = 7.6 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 3.73 (q, J = 6.8 Hz, 1H), 3.63 (s, 3H), 1.50 (d, J = 7.2 Hz, 3H), 1.32 (s, 12H); 13C NMR (100 MHz, CDCl3) δ 174.72, 143.73, 135.18, 134.97, 126.89, 124.98, 83.76, 52.02, 45.61, 24.85, 18.50; IR (KBr) νmax 2980.90, 1740.69 cm-1; MS: m/z 291.1 (M + H)+.
References:
Bhatthula, Bharath kumar goud;Kanchani, Janardhan reddy;Arava, Veera reddy;Subha [Tetrahedron,2019,vol. 75,# 7,p. 874 - 887]

74-88-4
342 suppliers
$15.00/10g

890839-11-9
21 suppliers
inquiry
39718-97-3
33 suppliers
$45.00/10mg

73183-34-3
562 suppliers
$6.00/5g

890839-11-9
21 suppliers
inquiry
2945-08-6
59 suppliers
inquiry

890839-11-9
21 suppliers
inquiry

50415-69-5
37 suppliers
$74.03/0.5g

890839-11-9
21 suppliers
inquiry