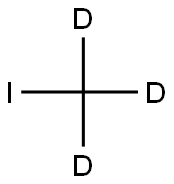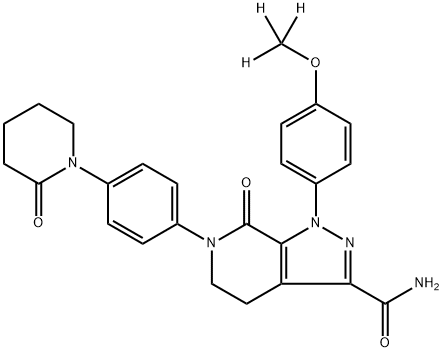
Apixaban-d3 synthesis
- Product Name:Apixaban-d3
- CAS Number:1131996-12-7
- Molecular formula:C25H25N5O4
- Molecular Weight:459.51
Yield: 38.61%
Reaction Conditions:
Stage #1:O-Demethylapixaban with potassium carbonate in acetonitrile at 20; for 0.166667 h;sealed tube;
Stage #2:iodomethane-d3 in acetonitrile at 80; for 6 h;sealed tube;
Steps:
2.2
Step 2; l-(4-A-Methoxyphenyl-7-oxo-6-(4-(2-oxo-l-piperidinyl) phenyl)- 4.5.6.7-tetrahydro-lH-pyrazole-r3. 4-cl pyridine-3-carboxamide:; In sealed tube, a mixture of l-(4-hydroxyphenyl)-7-oxo-6-(4-(2-oxopiperdin-l-yl)phenyl)-4,5,6,7- tetrahydro-lH-pyrazolo[3,4-c]pyridine-3-carboxamide (0.250 g, 0.561 mmol), dry acetonitrile, and potassium carbonate (0.154 g, 1.11 mmol) was stirred at ambient temperature for about 10 minutes. d3-Methyl iodide (97.15 mg, 0.67mmol) was then added. The mixture was heated at about 80 0C for about 6 hours, cooled to ambient temperature, and the solids removed by filtration. The resulting filtrate was concentrated and the resulting pale yellow liquid was purified by preparative ΗPLC on a KROMASIL 100 C-18 (30 x 250 mm) 5μ column (0.01M ammonium acetate / methanol (1 : 1); flow rate 20 mL / min; T / %B: 0 / 30, 10 / 80, 20 / 80, 20.1 / 30; UV: 210 nm; run time = 11.212 minutes). The title compound was purified as an off-white solid (100 mg, yield = 38.61%). mp: 236-240 0C; 1H NMR (400 MHz, CDCl3) δ 1.92-1.94 (m, 4H), 2.53-2.55 (m, 2H), 3.37 (t, J= 7.0, 2H), 3.58-3.59 (m, 2H), 4.11 (t, J= 6.8 Hz, 2H), 5.47 ( br s, IH), 6.84 ( br s, IH), 6.93 (d, J= 9.2 Hz, 2H), 7.25 (d, J= 8.0 Hz, 2H) , 7.33 (d, J= 8.4 Hz, 2H), 7.47 (d, J= 9.2 Hz, 2H); IR (KBr) υ 3454. 3307, 3259, 3180, 3060, 3010, 2949, 2864, 2223, 2074, 1675, 1616, 1510, 1459, 1409, 1298, 1262, 1217, 1155, 1104, 1017, 989 cm"1; MS 463 (M + 1).
References:
AUSPEX PHARMACEUTICALS, INC.;GANT, Thomas, G.;SHAHBAZ, Manoucherhr WO2010/30983, 2010, A2 Location in patent:Page/Page column 34
![1-(4-Hydroxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide](/CAS/20180703/GIF/503612-76-8.gif)