
Apixaban Impurity 51 synthesis
- Product Name:Apixaban Impurity 51
- CAS Number:1620494-29-2
- Molecular formula:C33H32N4O5
- Molecular Weight:564.63
545445-44-1
435 suppliers
$7.00/1g
![Acetic acid, 2-chloro-2-[2-[4-(phenylmethoxy)phenyl]hydrazinylidene]-, ethyl ester, (2Z)-](/CAS/20210305/GIF/1620494-28-1.gif)
1620494-28-1
1 suppliers
inquiry

1620494-29-2
18 suppliers
inquiry
Yield:1620494-29-2 54 mg
Reaction Conditions:
with triethylamine;potassium iodide in ethyl acetate at 25 - 80;
Steps:
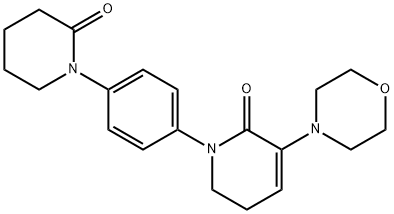
XX Preparation of 1-(4-benzyloxy-phenyl)-7-oxo-6-[4-(2-oxo-piperidin-1- yl)-phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester
In a clean round bottomed flask ethyl acetate (525ml), ethyl (2Z)-{[4-(benzyloxy)phenyl]hydrazono}(chloro)acetate (35gm), potassium iodide (1.73 g), 3-morpholino-1-[4-(2-oxopiperidin-1-yl)phenyl]-5,6-dihydropyridin-2(1H)-one (31.82g) and triethylamine (42.63g) were added at about 25-30°C . The reaction mass was heated to about 75-80°C for about 10-15 hrs. After completion, the reaction, mass was cooled to about 0 - 5°C and dilute hydrochloric acid was added slowly to the reaction mass. The temperature of the reaction mass was raised to about 25-30°C and stirred for 5-7 hrs. Ethyl acetate and water were added to the reaction mass and stirred for 15 min. The layers were separated and the organic layer was washed with aq. sodium carbonate solution followed by water and brine solution. The organic layer was concentrated under vacuum and cooled to about 25-30°C. Di isopropyl ether was added to the reaction mass and stirred for 1 hr. The precipitated solid was filtered and washed with di isopropyl ether. The solid was dried under vacuum at 45-50°C for 12hrs to obtain 54gm of 1-(4-benzyloxy-phenyl)-7-oxo-6-[4-(2-oxo-piperidin-1- yl)-phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxylic acid ethyl ester.
References:
WO2014/111954,2014,A1 Location in patent:Paragraph 0155
100-02-7
5 suppliers
$11.00/5G

1620494-29-2
18 suppliers
inquiry

100-39-0
425 suppliers
$10.00/10g

1620494-29-2
18 suppliers
inquiry

1145-76-2
105 suppliers
$26.00/5g

1620494-29-2
18 suppliers
inquiry
6373-46-2
160 suppliers
$6.00/1g

1620494-29-2
18 suppliers
inquiry