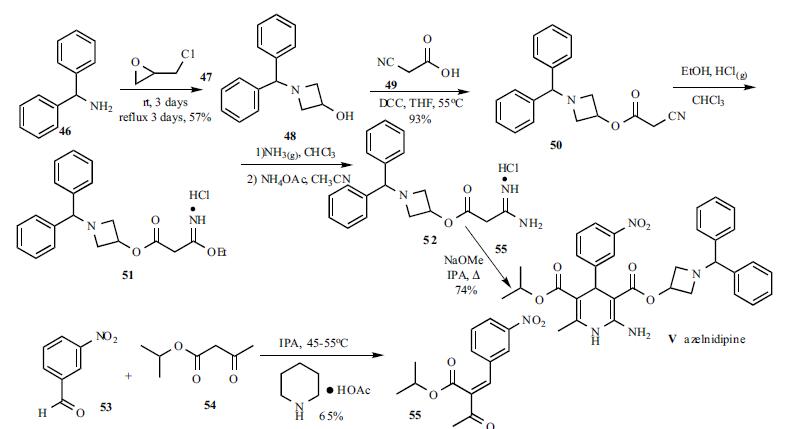
Azelnidipine synthesis
- Product Name:Azelnidipine
- CAS Number:123524-52-7
- Molecular formula:C33H34N4O6
- Molecular Weight:582.65
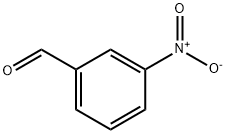
99-61-6
629 suppliers
$5.00/10g

123524-52-7
348 suppliers
$14.00/100mg
Yield:-
Steps:
Multi-step reaction with 4 steps
1: acetic acid; piperidine / ethanol / 24 h / 20 - 30 °C
2: potassium hydroxide / ethanol; water / 6 h / Reflux
3: dicyclohexyl-carbodiimide / tetrahydrofuran / 10 h / 60 - 70 °C
4: sodium methylate / ethanol / 6 h / Reflux
References:
WEIHAI DISU PHARM CO LTD;Weihai Dijia Pharmaceutical Co., Ltd.;GUAN, XITAO;LIANG, SONGJUN;CAO, DEQIANG;LI, LULU;MIAO, HUAMING CN105461691, 2016, A
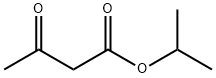
542-08-5
243 suppliers
$14.00/5g

123524-52-7
348 suppliers
$14.00/100mg
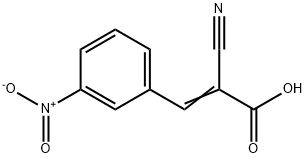
5468-46-2
0 suppliers
inquiry

123524-52-7
348 suppliers
$14.00/100mg