
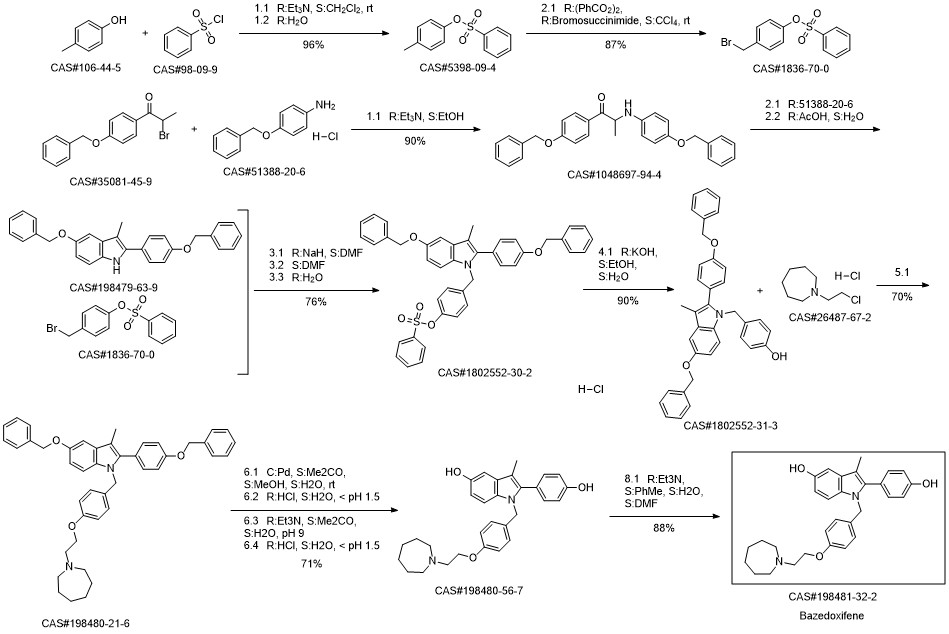
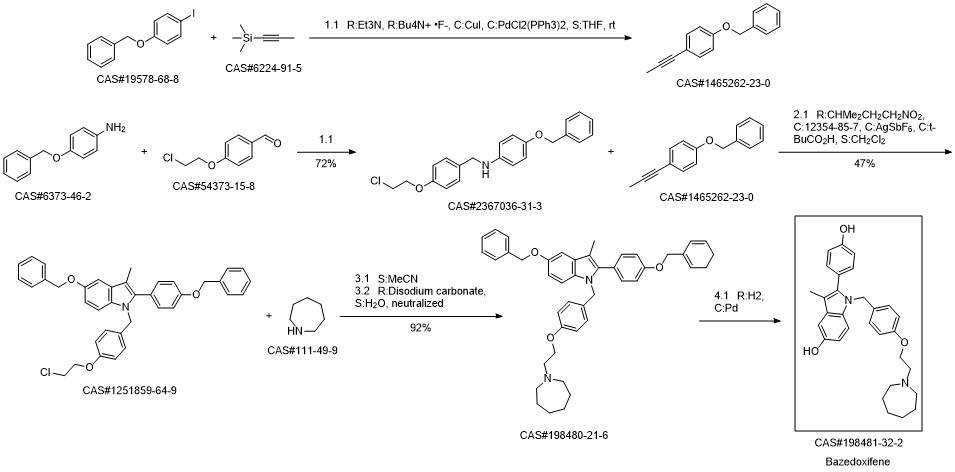
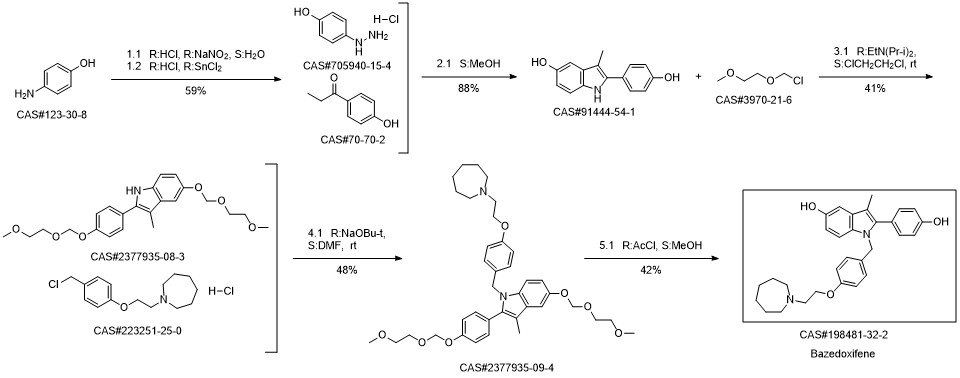
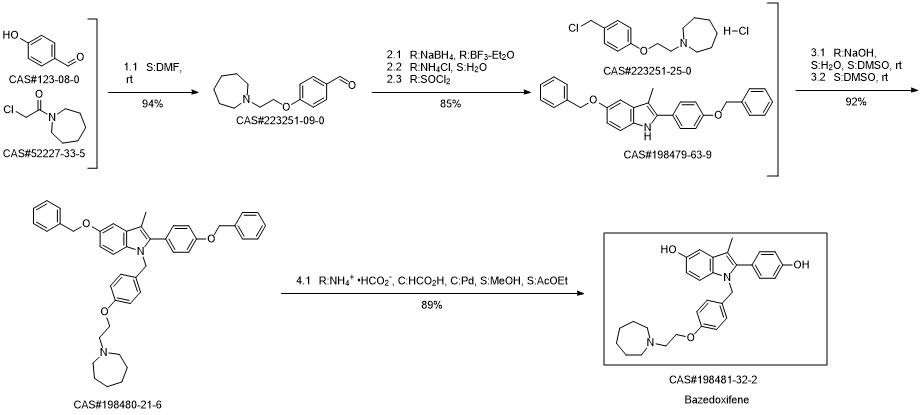
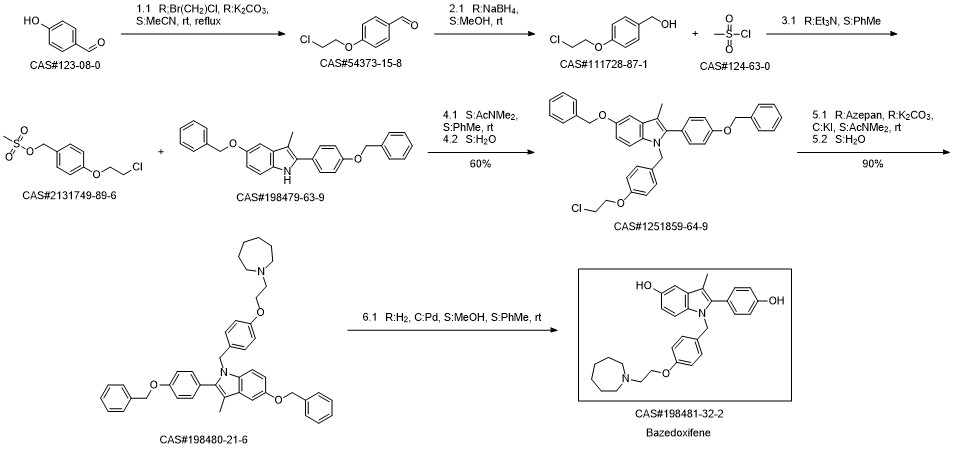
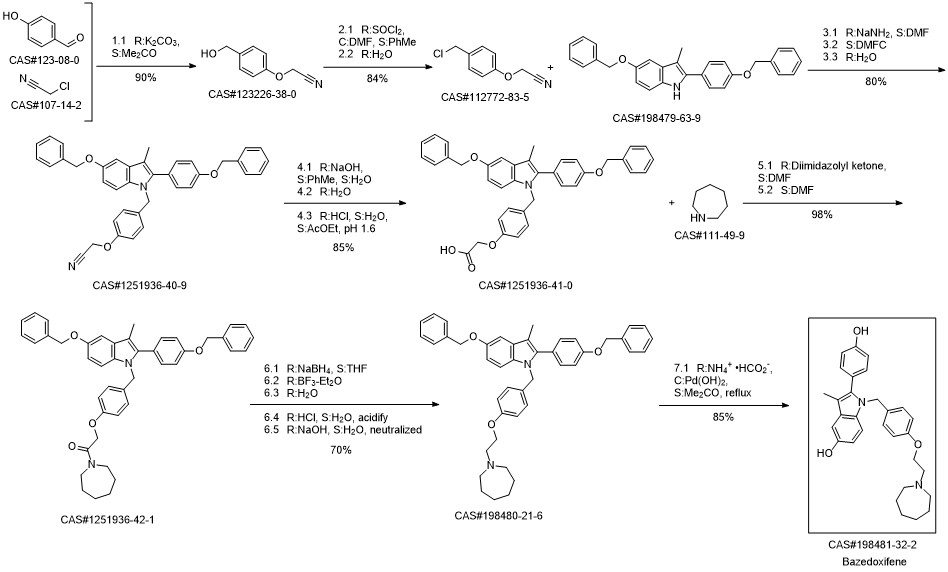
Bazedoxifene synthesis
- Product Name:Bazedoxifene
- CAS Number:198481-32-2
- Molecular formula:C30H34N2O3
- Molecular Weight:470.6
Mylavarapu, Ravikumar; Kondaiah, G. C. M.; Reddy, L. Amaranth; Dubey, Manoj Kumar; Jasmine; Mukkanti, Kagga; Bandichhor, Rakeshwar. An improved synthesis of Bazedoxifene acetate. Chemistry & Biology Interface. Volume 5. Issue 2. Pages 137-150. Journal; Online Computer File. (2015).
![1-[4-(2-AZEPAN-1-YL-ETHOXY)-BENZYL]-5-BENZYLOXY-2-(4-BENZYLOXY-PHENYL)-3-METHYL-1H-INDOLE](/StructureFile/ChemBookStructure20/GIF/CB81009554.gif)
198480-21-6
75 suppliers
$29.00/100mg

198481-32-2
125 suppliers
inquiry
Yield:198481-32-2 100%
Reaction Conditions:
with palladium on activated charcoal;hydrogen;sodium hydroxide in ethanol;ethyl acetateSolvent;Reagent/catalyst;
Steps:
1; 6.1 The specific synthesis steps are
Add in 2000mL single-necked bottle1- (4- (2- (Acheptan-1-yl) ethoxy) benzyl) -5- (benzyloxy) -2- (4- (benzyloxy) phenyl) -3- Methyl-1H-indole175.0g, dissolved with 1400mL of ethyl acetate and ethanol mixture, then added 5ml of 1.0mol / L sodium hydroxide, and then added 52.5g of palladium on carbon,Fully reacted under hydrogen, after TLC detected the reaction,Filter to remove the catalyst palladium carbon, and then add a certain amount of L-ascorbic acid to the filtrate,Remove the solvent under reduced pressure and dry,That is, 126 g of bazedoxifene free base was obtained with a yield of 100%.
References:
Nanjing Zheng Ji Pharmaceutical Co., Ltd.;Ge Min;Gu Wen;Wang Huaiqiu;Hu Chunchen;Li Lingchao CN111004165, 2020, A Location in patent:Paragraph 0028-0031; 0044-0046
![1H-Azepine-2,7-dione, tetrahydro-1-[2-[4-[[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indol-1-yl]methyl]phenoxy]ethyl]-](/CAS/20210305/GIF/1251936-45-4.gif)
1251936-45-4
0 suppliers
inquiry

198481-32-2
125 suppliers
inquiry
![4-[2-(1-AZEPANYL)ETHOXY]BENZYL CHLORIDE HCL](/CAS2/GIF/223251-25-0.gif)
223251-25-0
172 suppliers
$10.00/1g
![1H-Indol-5-ol, 2-[4-(acetyloxy)phenyl]-3-methyl-, 5-acetate](/CAS/20210111/GIF/91444-55-2.gif)
91444-55-2
0 suppliers
inquiry

198481-32-2
125 suppliers
inquiry
![4-[2-(1-AZEPANYL)ETHOXY]BENZYL CHLORIDE HCL](/CAS2/GIF/223251-25-0.gif)
223251-25-0
172 suppliers
$10.00/1g

91444-54-1
13 suppliers
inquiry

198481-32-2
125 suppliers
inquiry

![1H-Indol-5-ol, 1-[[4-(2-aminoethoxy)phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-](/CAS/20210305/GIF/1251936-44-3.gif)