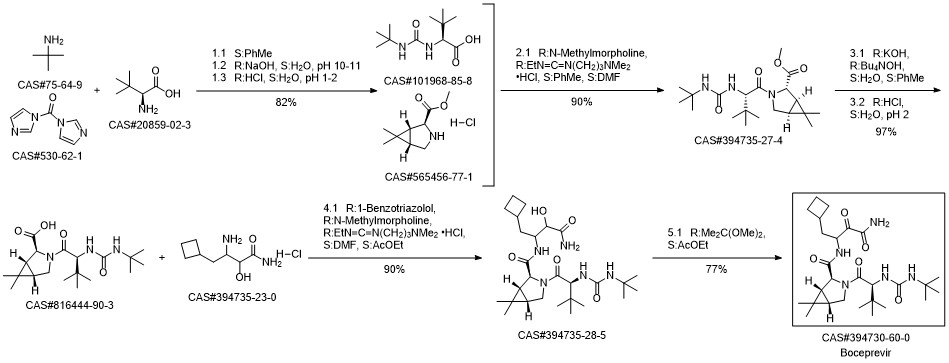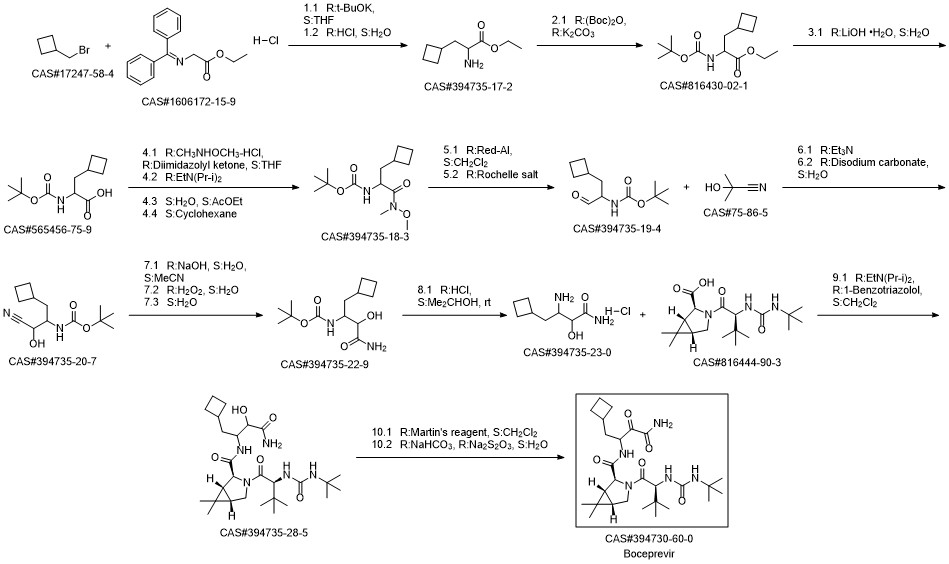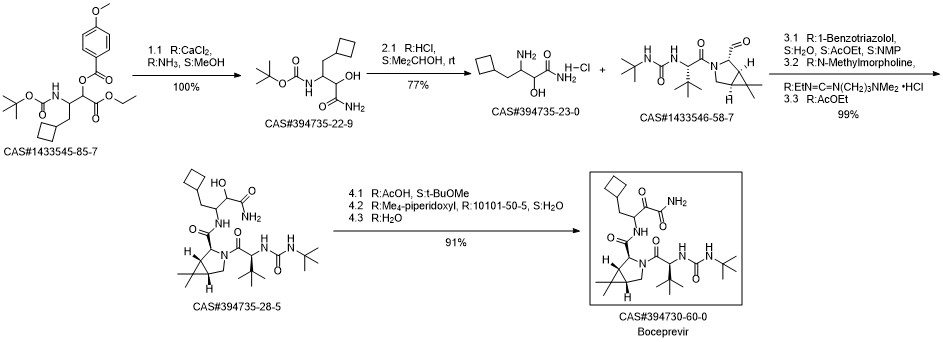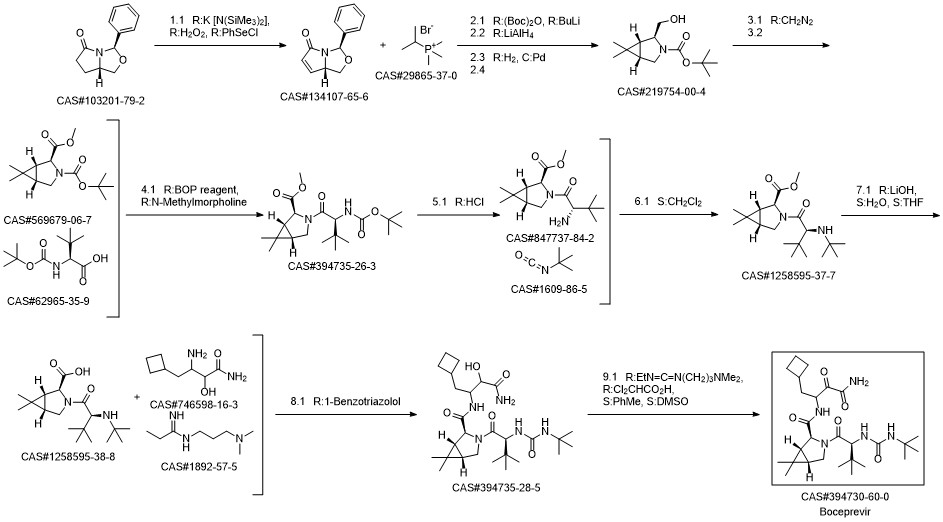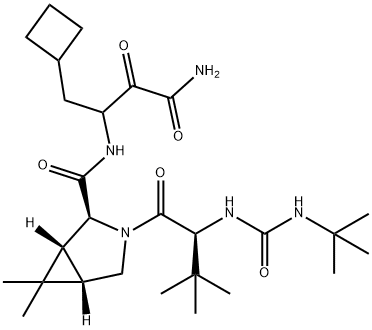
Boceprevir synthesis
- Product Name:Boceprevir
- CAS Number:394730-60-0
- Molecular formula:C27H45N5O5
- Molecular Weight:519.68
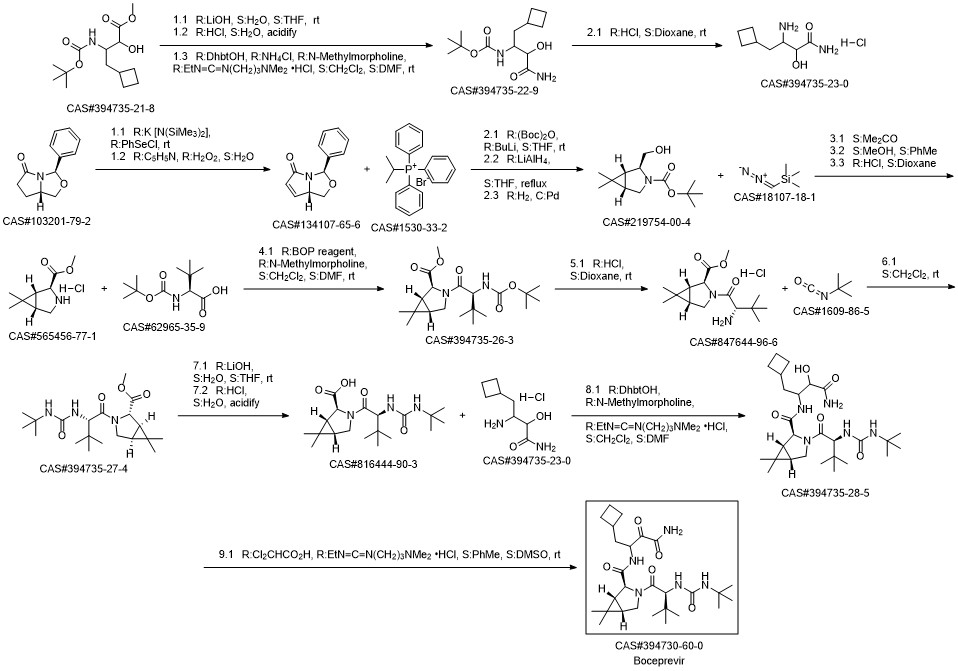
Venkatraman, Srikanth; Bogen, Stephane L.; Arasappan, Ashok; Bennett, Frank; Chen, Kevin; Jao, Edwin; Liu, Yi-Tsung; Lovey, Raymond; Hendrata, Siska; Huang, Yuhua; Pan, Weidong; Parekh, Tejal; Pinto, Patrick; Popov, Veljko; Pike, Russel; Ruan, Sumei; Santhanam, Bama; Vibulbhan, Bancha; Wu, Wanli; Yang, Weiying; Kong, Jianshe; Liang, Xiang; Wong, Jesse; Liu, Rong; Butkiewicz, Nancy; Chase, Robert; Hart, Andrea; Agrawal, Sony; Ingravallo, Paul; Pichardo, John; Kong, Rong; Baroudy, Bahige; Malcolm, Bruce; Guo, Zhuyan; Prongay, Andrew; Madison, Vincent; Broske, Lisa; Cui, Xiaoming; Cheng, Kuo-Chi; Hsieh, Tony Y.; Brisson, Jean-Marc; Prelusky, Danial; Korfmacher, Walter; White, Ronald; Bogdanowich-Knipp, Susan; Pavlovsky, Anastasia; Bradley, Prudence; Saksena, Anil K.; Ganguly, Ashit; Piwinski, John; Girijavallabhan, Viyyoor; Njoroge, F. George. Orally Bioavailable Hepatitis C Virus NS3 Protease Inhibitor: A Potential Therapeutic Agent for the Treatment of Hepatitis C Infection. Journal of Medicinal Chemistry. Volume 49. Issue 20. Pages 6074-6086. Journal. (2006).
![3-Azabicyclo[3.1.0]hexane-2-carboxaMide, N-[3-aMino-1-(cyclobutylMethyl)-2-hydroxy-3-oxopropyl]-3-[(2S)-2-[[[(1,1 -diMethylethyl)aMino]carbonyl]aMino]-3,3-diMethyl-1-oxobutyl]-6,6-diMe thyl-, (1R,2S,5S)-](/CAS/20150408/GIF/394735-28-5.gif)
394735-28-5
8 suppliers
inquiry

394730-60-0
196 suppliers
$28.00/1mg
Yield:394730-60-0 94%
Reaction Conditions:
Stage #1: (1R,2S,5S)-N-(4-amino-1-cyclobutyl-3-hydroxy-4-oxobutan-2-yl)-3-((S)-2-(3-(tert-butyl)ureido)-3,3-dimethylbutanoyl)-6,6-dimethyl-3-azabicylo[3.1.0]-hexane-2-carboxamidewith sodium hypochlorite solution;sodium acetate;potassium bromide;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical in tert-butyl methyl ether at 10 - 20;
Stage #2: with water;acetic acid in tert-butyl methyl ether; for 2.25 h;
Stage #3: with sodium hypochlorite solution;ascorbic acidProduct distribution / selectivity;more than 3 stages;
Steps:
1; 2 Preparation of Compound 2 Using Aqueous Acetic Acid in the Reaction Mixture
Example 1 Preparation of Compound 2 Using Aqueous Acetic Acid in the Reaction Mixture Into a 1 L, three necked flask is placed KBr (10 g, 84 mmol), NaOAc (10 g, 122 mmol), Compound 1 (50 g, 96 mmol), and TEMPO (15 g, 96 mmol), followed by 500 mL of MTBE. The reaction mixture is stirred at 350-400 rpm and the temperature is maintained at a temperature of from 10° C. to 20° C. Acetic acid (50 mL, 874 mmol), and water (5 mL) are added to the reaction mixture and the two phase mixture is agitated for 15 minutes. Continuously, over a two hour period, to the reaction mixture is added 158 mL of a 0.82 M solution of NaOCl (130 mmol). When all of the NaOCl solution is added, the reaction mixture is stirred for an additional 3 hours while maintaining the temperature. Water (50 mL) is added. The layers are separated and the organic layer is washed twice with water (2×250 mL). A solution of ascorbic acid, which is prepared from 50 g of sodium ascorbate, 200 mL of water, and 50 mL of 4N HCl, is added to the organic layer and the mixture is stirred for about 1 hour. After the layers are separated, the organic layer is washed twice with water (2×250 mL). The organic layer is concentrated by distilling off solvent at low temperature (0-5° C.) until the total volume is about 350 mL. The concentrated organic layer is added dropwise over 30 minutes into a 3 L flask containing 2 L of n-heptane at about 0° C. providing a white precipitate. The white precipitate is collected by filtration, washed with n-heptane (400 mL) and dried in a vacuum oven (2 hr at 25° C., 8 hr at 350, and 8° C. at 45° C.). The product is obtained as a white powder (typically 94-96% yield). 1H NMR, δ 0.84 (d, J=2.3 Hz, 3H), 0.90-1.02 (m, 9H), 0.99 (d, J=4.0 Hz, 3H), 1.24 (s, 9H), 1.40-1.86 (m, 7H), 1.90-2.10 (m, 3H), 2.25-2.40 (m, 1H), 3.75 (dd, J=5.3 and 10.4 Hz, 1H), 4.10 (dd, J=6.8 and 10.4 Hz, 1H), 4.4 (dd, J=3.0 and 5.3 Hz, 2H), 5.17 (dddd, J=4.6, 8.1, 8.1, and 10.4 Hz, 1H), 5.3 (br s, 2H), 6.71 (d, J=14.7 Hz, 1H), 6.90 (dd, J=2.3 and 19.0 Hz, 1H), and 7.34 (dd, J=7.1 and 20.2 Hz, 1H).; Example 2 Preparation of Compound 2 Using Glacial Acetic Acid in the Reaction Mixture Into a 2 L, three necked flask was charged KBr (20 g, 168 mmol), NaOAc (20 g, 243 mmol), Compound 1 (100 g, 192 mmol), and TEMPO (30 g, 192 mmol), followed by 800 mL of MTBE. The reaction mixture was stirred at 350400 rpm while the temperature of the reaction mixture was maintained at a temperature of from 10° C. to 20° C. Acetic acid (70 mL, 1223 mmol, used as received), was added and the mixture was agitated for 15 minutes additional. Continuously, over a two hour period, 315 ml of a 0.73M solution of NaOCl (230 mmol) was added to the reaction mixture. When all of the NaOCl solution had been added, agitation was continued for an additional 3 hours. Water (100 mL) was added to the reaction mixture at the end of 3 hours. The layers were separated and the organic layer was washed once with water (500 mL). A solution of ascorbic acid, which was prepared from 100 g of sodium ascorbate, 456 mL of water, and 44 mL of 36% HCl, was added to the organic layer and the mixture was stirred for about 2 hours. The layers were separated and then a solution of 3.5N HCL was added and stirred about 30 minutes. After the layers were separated, the organic layer was washed three times with water (3×500 mL). This organic layer was then added drop-wise over 30 minutes into a 5 L flask containing 3 L of n-heptane at about -10 to about 0° C. The white precipitate was filtered, washed with n-heptane (600 mL) and dried in a vacuum oven (2 hr at 25° C., 8 hr at 350, and 8° C. at 45° C.). The product was obtained as a white powder (93% yield). 1H NMR, δ 0.84 (d, J=2.3 Hz, 3H), 0.90-1.02 (m, 9H), 0.99 (d, J=4.0 Hz, 3H), 1.24 (s, 9H), 1.40-1.86 (m, 7H), 1.90-2.10 (m, 3H), 2.25-2.40 (m, 1H), 3.75 (dd, J=5.3 and 10.4 Hz, 1H), 4.10 (dd, J=6.8 and 10.4 Hz, 1H), 4.4 (dd, J=3.0 and 5.3 Hz, 2H), 5.17 (dddd, J=4.6, 8.1, 8.1, and 10.4 Hz, 1H), 5.3 (br s, 2H), 6.71 (d, J=14.7 Hz, 1H), 6.90 (dd, J=2.3 and 19.0 Hz, 1H), and 7.34 (dd, J=7.1 and 20.2 Hz, 1H).
References:
US2007/149459,2007,A1 Location in patent:Page/Page column 6-7

394735-19-4
17 suppliers
$65.00/5mg

394730-60-0
196 suppliers
$28.00/1mg
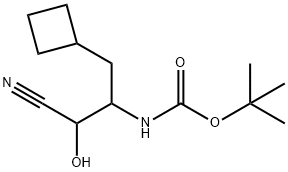
394735-20-7
17 suppliers
inquiry

394730-60-0
196 suppliers
$28.00/1mg
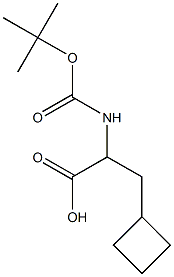
565456-75-9
39 suppliers
$91.67/0.5g

394730-60-0
196 suppliers
$28.00/1mg
![[1-(CyclobutylMethyl)-2-(MethoxyMethylaMino)-2-oxoethyl]-carbaMic Acid 1,1-DiMethylethyl Ester](/CAS/GIF/394735-18-3.gif)
394735-18-3
11 suppliers
$625.00/1mg

394730-60-0
196 suppliers
$28.00/1mg