
Enzalutamide synthesis
- Product Name:Enzalutamide
- CAS Number:915087-33-1
- Molecular formula:C21H16F4N4O2S
- Molecular Weight:464.44
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g
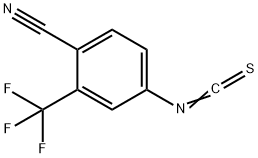
143782-23-4
423 suppliers
$60.00/1g

915087-33-1
498 suppliers
$5.00/1mg
Yield:915087-33-1 88.5%
Reaction Conditions:
Stage #1:2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methyl propanoic acid with triethylamine in dichloromethane;toluene at 30; for 0.166667 h;
Stage #2:4-isothiocyanato 2-(trifluoromethyl)benzonitrile in toluene at 50 - 60; for 4 h;Temperature;Solvent;
Steps:
5 Example 5: Preparation of Enzalutamide:
The compound 9 (60.0 g, 236.1 nmol) obtained in Example 2, triethylamine (24.0 g, 237.6 nmol), 180 mL of toluene, and 120 mL of dichloromethane were added to the reaction flask, and stirred at 30°C for 10 minutes.Heat to 50°C to dissolve and clear.A solution of 4-isothiocyano-2-(trifluoromethyl)benzonitrile (compound 7, 81.0 g, 355.3 nmol) in toluene (300 ml) was slowly added dropwise.After dripping, the temperature was raised to 60°C and the reaction was continued for 4 hours.TLC (dichloromethane: methanol = 50:1) dot plate to track the reaction.After the reaction, 240 mL of methanol was added to make the solution clear, 6.0 g of activated carbon was added, and the color was decolorized for 30 minutes.Concentrate to dryness, add 200 mL of dichloromethane to the oily residue, cool to 0-5°C, and stir for 1 hour.After filtration, the filtrate was washed successively with 240 mL of saturated brine and 60 mL of 10% aqueous hydrochloric acid.The water layer was separated, and the organic layer was dried over anhydrous sodium sulfate and concentrated to dryness.Add 240 mL of methanol to the residue and heat to reflux to make it clear.Slowly lower the temperature to 60°C and keep it warm for 1 hour.Cool to 0-5°C and continue stirring for 12 hours.After filtering and vacuum drying at 40°C for 5 hours, 97.0 g of white crystals were obtained, the yield was 88.5%, the HPLC purity was 99.6%, and the impurity A was 0.03%.
References:
Anlite (Shanghai) Pharmaceutical Technology Co., Ltd.;Jiangsu Chuangnuo Pharmaceutical Co., Ltd.;Shanghai Chuangnuo Pharmaceutical Group Co., Ltd.;Zhou Wu;Hu Meng CN112047888, 2020, A Location in patent:Paragraph 0116; 0143-0144; 0147-0148
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine methyl ester](/CAS/20150408/GIF/1332524-01-2.gif)
1332524-01-2
151 suppliers
inquiry

143782-23-4
423 suppliers
$60.00/1g

915087-33-1
498 suppliers
$5.00/1mg
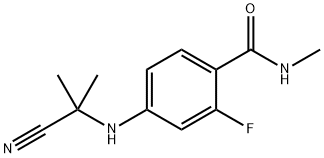
915087-32-0
102 suppliers
inquiry

143782-23-4
423 suppliers
$60.00/1g

915087-33-1
498 suppliers
$5.00/1mg
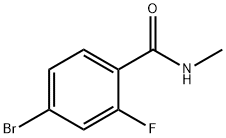
749927-69-3
308 suppliers
$40.00/1 g

143782-28-9
1 suppliers
inquiry

915087-33-1
498 suppliers
$5.00/1mg
![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine](/CAS/20150408/GIF/1289942-66-0.gif)
1289942-66-0
232 suppliers
$11.00/1g

915087-33-1
498 suppliers
$5.00/1mg