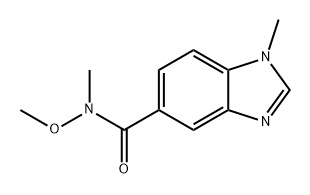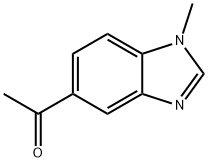
Ethanone, 1-(1-methyl-1H-benzimidazol-5-yl)- (9CI) synthesis
- Product Name:Ethanone, 1-(1-methyl-1H-benzimidazol-5-yl)- (9CI)
- CAS Number:265107-91-3
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
Yield:265107-91-3 87.59%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at 0 - 20; for 3.25 h;Inert atmosphere;
Steps:
Synthesis of 1-(1-methyl-1H-benzo[d]imidazol-5-yl)ethan-1-one (174)
To a stirred solution of compound 173 (8.1 g, 36.95 mmol) in anhydrous THF (80 mL) under inert atmosphere was added methyl magnesium bromide (24.77 mL, 73.97mmol, 3 M sol. in diethyl ether) dropwise for 15 min at 0 oC, followed by warming to room temperature and stirring for 3 h. The reaction was monitored by TLC. After completion of the reaction, the reaction mixture was quenched with saturated ammonium chloride solution (50 mL) and extracted withethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated in vacuo.The crude compound was purified by silica gel column chromatography using 2% MeOH/DCM to afford the title compound 174 (5.65 g, 87.59%) as an off white solid. TLC: 5% MeOH/DCM (Rf: 0.4); 1H NMR (400 MHz, DMSO-d6): δ 8.34- 8.32 (m, 2H), 7.90 (d, J = 8.4 Hz, 1H), 7.66 (d, J = 8.8 Hz, 1H), 3.87 (s, 3H), 2.64 (s, 3H); LCMS Calculated for C10H10N2O: 174.08; Observed: 175.10 (M+1)+.
References:
WO2018/160878,2018,A1 Location in patent:Page/Page column 342
906352-57-6
15 suppliers
$90.00/250mg

265107-91-3
13 suppliers
inquiry
53484-17-6
77 suppliers
$45.00/100mg

265107-91-3
13 suppliers
inquiry