
MI-192 synthesis
- Product Name:MI-192
- CAS Number:1415340-63-4
- Molecular formula:C24H21N3O2
- Molecular Weight:383.44

463-49-0
52 suppliers
$125.00/10g
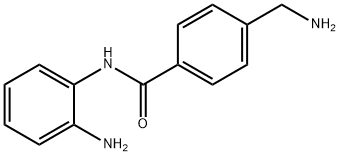
394253-88-4
4 suppliers
inquiry
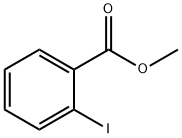
610-97-9
430 suppliers
$9.00/1g

1415340-63-4
36 suppliers
$39.00/1mg
Yield:1415340-63-4 65%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);trifuran-2-yl-phosphane;potassium carbonate in N,N-dimethyl-formamide at 80; under 413.729 Torr; for 25.5 h;Schlenk technique;Time;
Steps:
7.3 Step 3-Preparation of N-(2-Amino-phenyl)-4-(4-methylene-1-oxo-3,4-dihydro-1H-isoquinolin-2-ylmethyl)-benzamide
DMF degassed by sparging with nitrogen for 1 hour. To a Schlenk tube was charged 2 (0.70 g, 2.901 mmol), methyl 2-iodobenzoate (0.68 g, 2.595 mmol, 0.9 equivalents), potassium carbonate (0.72 g, 5.209 mmol, 1.8 equivalents), tri(2-furyl)phosphine (0.067 g, 0.289 mmol, 10 mol %), tris(dibenzylideneacetone)dipalladium(0) (0.066 g, 0.072 mmol, 2.5 mol %) and DMF (70 ml). The Schlenk tube was sealed, then evacuated and backfilled with nitrogen twice. It was then left open to vacuum for 2 minutes. A cylinder of allene was set to 8 psi, the Schlenk tube isolated from vacuum and then opened (whilst still under vacuum) to the allene cylinder, and left open to it for 1 minute. The Schlenk tube was sealed and heated to 80° C. for 21 hours (behind a blast shield). The reaction was left to cool to ambient, then vented. LCMS analysis showed >99% conversion of the methyl 2-iodobenzoate. The Schlenk tube was evacuated and backfilled with nitrogen twice to remove residual allene. The reaction mixture was poured into 3% sodium chloride solution (300 ml) and extracted with ethyl acetate (2×100 ml). The ethyl acetate layers were combined and washed with 3% sodium chloride solution (100 ml), saturated disodium citrate solution (30 ml), 0.1M EDTA disodium salt solution (50 ml) and 3% sodium chloride solution (50 ml). The organics were dried over magnesium sulphate and filtered. To the filtrate was added activated charcoal (0.07 g) and stirred for 35 minutes. The charcoal was removed by filtration through Kieselguhr and the filtrate concentrated under reduced pressure to give crude 3, 1.10 g (99%). This was then columned using a Combiflash Rf (120 g silica column, DCM/MeOH) to give 3, 0.58 g (52%). [0287] This procedure carried out on 4 more batches, the results are summarised in the following table. [TABLE-US-00002] Input/g Time/hours Yield/g 0.70 21 0.64(58%) 0.60 23 0.55(58%) 0.60 25 0.62(65%) 0.56 24 0.53(60%) [0288] The 5 batches were combined, dissolved in a mixture of DCM and methanol and the resulting clear solution concentrated under reduced pressure to give 3, 2.87 g. [0289] LCMS: Purity 99% Retention time 5.6 minutes MI:M+1=384 [0290] NMR (CDCl3): 8.23 (m, 1H), 7.97 (s, b, 1H), 7.90 (d, J=8 Hz), 7.60-7.44 (m, 5H), 7.36 (d, J=8 Hz, 1H), 7.11 (m, 1H), 6.87 (m 2H), 5.61 (t, J=1 Hz), 5.19 (t, J=2 Hz, 1H), 4.89 (s, 2H), 4.17 (t, J=1 Hz, 2H), 3.90 (s, 2H). [0291] NMR (d6-DMSO): 9.65 (s, 1H), 8.03 (dd, J=1 Hz, 7 Hz, 1H), 7.97 (d, J=8 Hz, 2H), 7.74 (d, J=7 Hz, 1H), 7.61 (m, 1H), 7.51 (m, 1H), 7.44 (d, J=8 Hz, 2H), 7.16 (d, J=8 Hz, 1H), 6.97 (m, 1H), 6.78 (dd, J=1 Hz, 8 Hz, 1H), 6.59 (m, 1H), 5.73 (s, 1H), 5.32 (s, 1H), 4.90 (s, 2H), 4.81 (s, 2H), 4.27 (s, 2H).
References:
US2014/135327,2014,A1 Location in patent:Paragraph 0285-0291
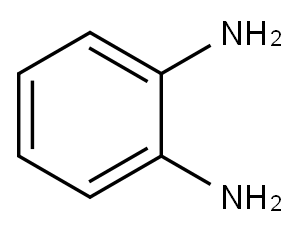
95-54-5
536 suppliers
$9.00/1g

1415340-63-4
36 suppliers
$39.00/1mg
![4-[(tert-Butoxycarbonylamino)methyl]benzoic acid](/CAS/GIF/33233-67-9.gif)
33233-67-9
166 suppliers
$7.00/1g

1415340-63-4
36 suppliers
$39.00/1mg
![Carbamic acid, N-[[4-[[(2-aminophenyl)amino]carbonyl]phenyl]methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/391244-48-7.gif)
391244-48-7
0 suppliers
inquiry

1415340-63-4
36 suppliers
$39.00/1mg