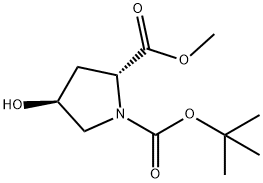
N-Boc-Trans-4-Hydroxy-D-proline methyl ester synthesis
- Product Name:N-Boc-Trans-4-Hydroxy-D-proline methyl ester
- CAS Number:135042-17-0
- Molecular formula:C11H19NO5
- Molecular Weight:245.27
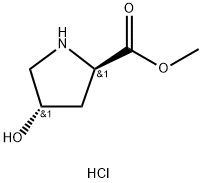
481704-21-6
112 suppliers
$11.00/250mg

24424-99-5
823 suppliers
$13.50/25G

135042-17-0
161 suppliers
$8.00/250mg
Yield:135042-17-0 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in 1,4-dioxane at 0 - 20; for 1 h;
Steps:
7
Add di-tert-butyl dicarbonate (11.2 g, 51.35 mmol) to a solution of 4-(S)- hydroxypyrrolidine-2- (R)-carboxylic acid methyl ester hydrochloride (7.17 g, 39.5 mmol) in 1,4-dioxane (80 mL). Cool in an ice bath and add N,N-diisopropylethylamine (11.7 mL, 67.2 mmol). Remove ice bath and stir 1 h at room temperature. Concentrate and dissolve in ethyl acetate, wash with 5% aqueous citric acid solution (2 x100 mL), water, saturated aqueous sodium bicarbonate, saturated aqueous sodium chloride, dry (magnesium sulfate), concentrate and purify (silica gel chromatography, eluting with hexanes and ethyl acetate) to give the title compound as a white solid (9.83 g, 100%). MS (ES): m/z = 246.1 [M+H].
References:
WO2005/108358,2005,A2 Location in patent:Page/Page column 38-39
![1,2-Pyrrolidinedicarboxylic acid, 4-[(4-nitrobenzoyl)oxy]-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4S)-](/CAS/20210305/GIF/361367-93-3.gif)
361367-93-3
0 suppliers
inquiry

135042-17-0
161 suppliers
$8.00/250mg
147266-92-0
169 suppliers
$60.00/100mg

74-88-4
342 suppliers
$15.00/10g

135042-17-0
161 suppliers
$8.00/250mg

24424-99-5
823 suppliers
$13.50/25G
178962-09-9
31 suppliers
inquiry

135042-17-0
161 suppliers
$8.00/250mg

147266-92-0
169 suppliers
$60.00/100mg
18107-18-1
210 suppliers
$33.00/5g

135042-17-0
161 suppliers
$8.00/250mg