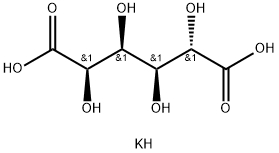
Potassium bisaccharate synthesis
- Product Name:Potassium bisaccharate
- CAS Number:576-42-1
- Molecular formula:C6H11KO8
- Molecular Weight:250.24
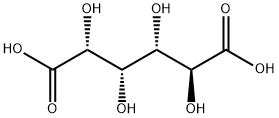
87-73-0
123 suppliers
inquiry

576-42-1
121 suppliers
$90.00/5 g
Yield:576-42-1 83.2 g
Reaction Conditions:
Stage #1:D-glucaric acid with potassium hydroxide in water
Stage #2: with hydrogenchloride in water; pH=3.4Cooling with ice;
Steps:
4.9. Monopotassium d-glucarate isolation from an oxidation where nitric acid was evaporatively removed at reduced pressure with heat
The pH of the concentrated reaction mixture from the oxidation of d-glucose (4:1 molar ratio of nitric acid to d-glucose) reconstituted with water (370 mL) was adjusted to a constant pH of 9.5 with 45% KOH. The solution was cooled in an ice bath and titrated to pH 3.4 with concentrated hydrochloric acid. A precipitate was formed when the solution pH dropped below 5. The mixture was cooled and held at 5 °C for 4 h and the precipitate then isolated by filtration. The resulting solid cake was washed with cold water and dried at reduced pressure for 18 h to give solid monopotassium d-glucarate (3), 83.2 g (45% yield from 134.3 g of dextrose).
References:
Smith, Tyler N.;Hash, Kirk;Davey, Cara-Lee;Mills, Heidi;Williams, Holly;Kiely, Donald E. [Carbohydrate Research,2012,vol. 350,p. 6 - 13] Location in patent:experimental part
492-62-6
198 suppliers
$6.00/25g

576-42-1
121 suppliers
$90.00/5 g

6556-12-3
277 suppliers
$22.39/5G

576-42-1
121 suppliers
$90.00/5 g
6027-89-0
105 suppliers
$50.00/500 mg

576-42-1
121 suppliers
$90.00/5 g

2280-44-6
8 suppliers
inquiry

576-42-1
121 suppliers
$90.00/5 g