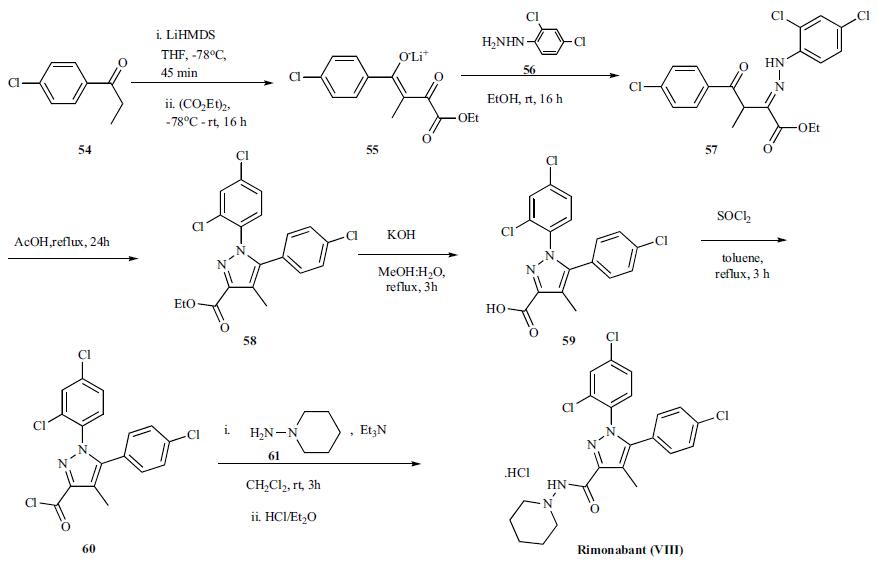
Rimonabant synthesis
- Product Name:Rimonabant
- CAS Number:168273-06-1
- Molecular formula:C22H21Cl3N4O
- Molecular Weight:463.79
158681-13-1
174 suppliers
$41.00/10mg

168273-06-1
249 suppliers
$42.00/5mg
Yield:168273-06-1 93%
Reaction Conditions:
with methanol;potassium hydroxide;water at 0 - 5; for 1 h;
Steps:
1
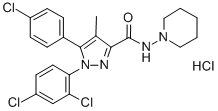
EXAMPLE 1; Solid Phase III A solution of KOH (10,6 g) in methanol (87 mL) are added to a solution of Rimonabant hydrochloride (86.87 g) in methanol (347 mL). During the addition the internal temperature is maintained at 0-5°C. Once the addition is completed, water (174 mL) is added. The resulting suspension is cooled to 0-5°C and stirred during 1hs to the mentioned temperature. The solid is filtered and washed with water (3 x 300 mL). The solid is dried under vacuum at 50-55°C until to constant weight. In this trial, 74.50 g of crude solid is obtained (Yield: 93%)
References:
Vértessy, Miklós EP1944297, 2008, A1 Location in patent:Page/Page column 5-6
2213-43-6
197 suppliers
$48.89/5g
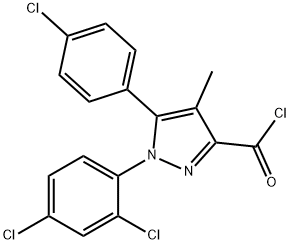
168273-05-0
2 suppliers
inquiry

168273-06-1
249 suppliers
$42.00/5mg
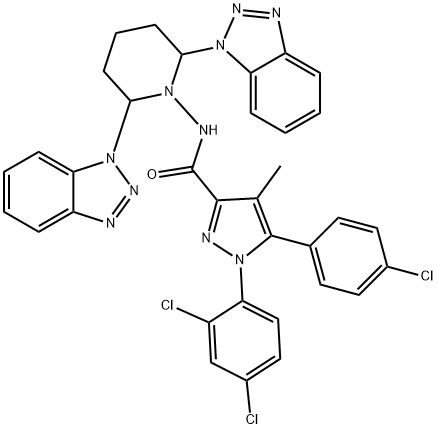
1040405-33-1
1 suppliers
inquiry

168273-06-1
249 suppliers
$42.00/5mg

2213-43-6
197 suppliers
$48.89/5g
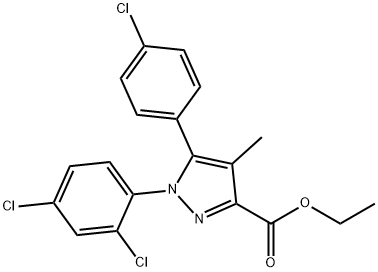
158941-22-1
13 suppliers
inquiry

168273-06-1
249 suppliers
$42.00/5mg
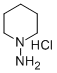
63234-70-8
105 suppliers
inquiry

158941-22-1
13 suppliers
inquiry

168273-06-1
249 suppliers
$42.00/5mg