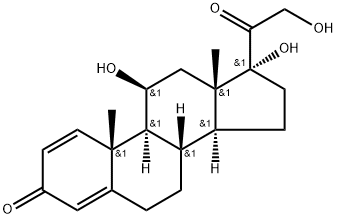
Prednisolone Solution
- CAS No.
- 50-24-8
- Chemical Name:
- Prednisolone Solution
- Synonyms
- Prednisolon;cortalone;delta-cortef;Prednisolone CRS;Hydrocortisone EP Impurity A;Prednisolone for system suitability CRS;deltaf;solone;steran;prednis
- CBNumber:
- CB00128026
- Molecular Formula:
- C21H28O5
- Molecular Weight:
- 360.45
- MDL Number:
- MFCD00003649
- MOL File:
- 50-24-8.mol
| Melting point | 240 °C (dec.) (lit.) |
|---|---|
| alpha | D25 +102° (dioxane) |
| Boiling point | 412.46°C (rough estimate) |
| Density | 1.0963 (rough estimate) |
| refractive index | 100 ° (C=1, Dioxane) |
| Flash point | 2℃ |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble in water, soluble in ethanol (96 per cent) and in methanol, sparingly soluble in acetone, slightly soluble in methylene chloride. It shows polymorphism (5.9). |
| pka | 12.46±0.70(Predicted) |
| form | powder |
| color | White to Off-White |
| Water Solubility | 2.225g/L(25 ºC) |
| Merck | 14,7721 |
| BRN | 1354103 |
| BCS Class | 1 |
| InChIKey | OIGNJSKKLXVSLS-VWUMJDOOSA-N |
| CAS DataBase Reference | 50-24-8(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | prednisolone |
| FDA UNII | 9PHQ9Y1OLM |
| NCI Drug Dictionary | Cortalone |
| ATC code | A01AC54,A07EA01,C05AA04,D07AA03,D07XA02,H02AB06,R01AD02,R01AD52,S01BA04,S01CB02,S02BA03,S03BA02 |
| EPA Substance Registry System | Prednisolone (50-24-8) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H360FD | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501 | |||||||||
| Hazard Codes | ||||||||||
| Hazardous Substances Data | 50-24-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in mouse: 1680mg/kg | |||||||||
| NFPA 704 |
|
Prednisolone Solution price More Price(43)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | P-121 | Prednisolone solution 100?μg/mL in acetonitrile, ampule of 1?mL, certified reference material, Cerilliant? | 50-24-8 | 1mL | $249 | 2024-03-01 | Buy |
| Sigma-Aldrich | BP464 | Prednisolone British Pharmacopoeia (BP) Assay Standard | 50-24-8 | 100MG | $223 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46656 | Prednisolone VETRANAL | 50-24-8 | 250mg | $53.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1555005 | Prednisolone United States Pharmacopeia (USP) Reference Standard | 50-24-8 | 200mg | $436 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001920 | Prednisone impurity B European Pharmacopoeia (EP) Reference Standard | 50-24-8 | Y0001920 | $149 | 2022-05-15 | Buy |
Prednisolone Solution Chemical Properties,Uses,Production
Overview
Prednisolone is a synthetic adrenocortical steroid drug with predominantly glucocorticoid properties[1, 2, 4, 10]. Prednisone is a white to practically white, odorless, crystalline powder and has a molecular weight of 358.43. Prednisone is very slightly soluble in water, slightly soluble in alcohol, chloroform, dioxane, and methanol[3]. Some of these properties reproduce the physiological actions of endogenous glucocorticosteroids, but others do not necessarily reflect any of the adrenal hormones' normal functions; they are seen only after administration of large therapeutic doses of the drug. The pharmacological effects of prednisolone which are due to its glucocorticoid properties include: promotion of gluconeogenesis; increased deposition of glycogen in the liver; inhibition of the utilization of glucose; anti-insulin activity; increased catabolism of protein; increased lipolysis; stimulation of fat synthesis and storage; increased glomerular filtration rate and resulting increase in urinary excretion of urate (creatinine excretion)[5-9].
Figure 1 the chemical structure of prednisolone;
Indication
It is indicated for the treatment of primary or secondary adrenocortical insufficiency[9], such as congenital adrenal hyperplasia, thyroiditis. It is also used to treat psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, bursitis, acute gouty arthritis and epicondylitis[10]; indicated for treatment of systemic lupus erythematosus, pemphigus and acute rhematic carditis; can be used in the treatment of leukemias, lymphomas, thrombocytopenia purpura and autoimmune hemolytic anemia; can be used to treat celiac disease, insulin resistance, ulcerative colitis and liver disorders.
Mechanism of action
Glucocorticoids such as Prednisolone can inhibit leukocyte infiltration at the site of inflammation, interfere with mediators of inflammatory response, and suppress humoral immune responses[10-13]. The antiinflammatory actions of glucocorticoids are thought to involve phospholipase A2 inhibitory proteins, lipocortins, which control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes[10-13]. Prednisolone reduces inflammatory reaction by limiting the capillary dilatation and permeability of the vascular structures[14]. These compounds restrict the accumulation of polymorphonuclear leukocytes and macrophages and reduce the release of vasoactive kinins. Recent research suggests that corticosteroids may inhibit the release of arachidonic acid from phospholipids, thereby reducing the formation of prostaglandins. Prednisolone is a glucocorticoid receptor agonist [15]. On binding, the cortico-receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes. The DNA bound receptor then interacts with basic transcription factors, causing an increase or decrease in expression of specific target genes, including suppression of IL2 (interleukin 2) expression.
Pharmacokinetics
Prednisolone is cleared from the body primarily by hepatic metabolism, and greater than 90% of radioactivity administered orally or intravenously as[4-14] prednisolone is recovered in the urine[16,17]. Only approximately 7-15% of an oral dose of prednisone or prednisolone is excreted as unchanged prednisolone in the urine, the remainder being recovered as a variety of metabolites[18, 19].
The plasma half-life of prednisolone ranges from 2.5 to 3.5 hr(1). Similar half-life values for prednisolone are observed after oral prednisolone is administered[20, 21]. Mean plasma half-lives were 4.0 hr and 5.0 hr for the 12and 48-mg doses, respectively. Plasma clearance averaged 98.5-ml/min/1.73 m 2 and 120.1-ml/min/1.73 m 2 after these doses. Neither half-life nor clearance was statistically different between the two dose levels. Values for volume of distribution were calculated to be 21.6 and 27.7 liters /1.73 m 2 for Vl and 12.1 and 31.1 liters/1.73 m 2 for V2 with the 12and 48-mg doses, respectively. Only the differences in V2 were found to be statistically significant. It appears that prednisolone may exhibit dose-dependent pharmacokinetics, so that, with increasing dose, values for volume of distribution, plasma clearance, and half-life may increase. Although the exact reasons for these changes have not been established, they are believed to be related to changes in the plasma protein binding of prednisolone. It has been shown that prednisolone binds to plasma proteins (transcortin and albumin) in a nonlinear manner over the range of doses used, so that the percentage unbound increases with increasing dose[22]. This then leads to the observed changes in the pharmacokinetic parameters of prednisolone.
Adverse reactions
Various adverse reactions may be associated with the use of prednisolone[1, 10].
Common adverse reactions for corticosteroids include fluid retention, alteration in glucose tolerance, elevation in blood pressure, behavioral and mood changes, increased appetite and weight gain.
Allergic Reactions: Anaphylaxis, angioedema.
Cardiovascular: Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis
Dermatologic: Acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scalp, edema, facial erythema, hyper or hypo-pigmentation, impaired wound healing, increased sweating, petechiae and ecchymoses, rash, sterile abscess, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria
Endocrine: Abnormal fat deposits, decreased carbohydrate tolerance, development of Cushingoid state, hirsutism, manifestations of latent diabetes mellitus and increased requirements for insulin or oral hypoglycemic agents in diabetics, menstrual irregularities, moon facies, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery or illness), suppression of growth in children
Fluid and Electrolyte Disturbances: Fluid retention, potassium loss, hypertension, hypokalemic alkalosis, and sodium retention
Gastrointestinal: Abdominal distention, elevation in serum liver enzymes levels (usually reversible upon discontinuation), hepatomegaly, hiccups, malaise, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, ulcerative esophagitis.
General: Increased appetite and weight gain
Metabolic: Negative nitrogen balance due to protein catabolism
Musculoskeletal: Osteonecrosis of femoral and humeral heads, charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, steroid myopathy, tendon rupture, vertebral compression fractures.
Neurological: Arachnoiditis, convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudo-tumor cerebri) usually following discontinuation of treatment, insomnia, meningitis, mood swings, neuritis, neuropathy, paraparesis/paraplegia, paresthesia, personality changes, sensory disturbances, vertigo.
Precaution
Alterations in Endocrine Function[10]
Corticosteroids can produce reversible hypothalamic-pituitary adrenal (HPA) axis suppression with the potential for corticosteroid insufficiency after withdrawal of treatment. Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. If the patient is receiving corticosteroids already, dosage may have to be increased.
Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently. Mineralocorticoid supplementation is of particular importance in infancy.
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.
Increased Risks Related to Infection[10]
Corticosteroids may increase the risks related to infections with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic infections. The degree to which the dose, route and duration of corticosteroid administration correlates with the specific risks of infection is not well characterized, however, with increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may mask some signs of infection and may reduce resistance to new infections.
Overdosage
The effects of accidental ingestion of large quantities of prednisone over a very short period of time have not been reported, but prolonged use of the drug can produce mental symptoms, moon face, abnormal fat deposits, fluid retention, excessive appetite, weight gain, hypertrichosis, acne, striae, ecchymosis, increased sweating, pigmentation, dry scaly skin, thinning scalp hair, increased blood pressure, tachycardia, thrombophlebitis, decreased resistance to infection, negative nitrogen balance with delayed bone and wound healing, headache, weakness, menstrual disorders, accentuated menopausal symptoms, neuropathy, fractures, osteoporosis, peptic ulcer, decreased glucose tolerance, hypokalemia, and adrenal insufficiency. Hepatomegaly and abdominal distention have been observed in children.
Treatment of acute overdosage is by immediate gastric lavage or emesis followed by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy the dosage of prednisone may be reduced only temporarily, or alternate day treatment may be introduced.
Tips
The following tips should be followed when you plan to/are using prednisolone[1]
You should not use prednisolone if you are allergic to it or have a fungal infection anywhere in your body.
Prednisolone has the potential weaken your immune system, making it easier for you to get an infection. Steroids can also worsen an infection you already have, or reactivate an infection you recently had. You should keep consulting your doctors about any illness or infection you have had within the past several weeks. To make sure prednisolone is safe for you, let your doctor know if you have ever had active tuberculosis, a thyroid disorder, herpes infection of the eyes, stomach ulcers, ulcerative colitis, or diverticulitis, depression, mental illness, or psychosis, liver disease (especially cirrhosis), high blood pressure, osteoporosis, a muscle disorder such as myasthenia gravis; or multiple sclerosis.
You should also inform your doctor if you have diabetes. Steroid medicines may further increase the glucose (sugar) levels in your blood or urine, worsening the situation of diabetes. You may also need to adjust the dose of your diabetes medications in that case. It is not known whether this medicine will harm an unborn baby. Tell your doctor if you are pregnant or plan to become pregnant. It is not known whether prednisolone passes into breast milk or if it could affect the nursing baby. Tell your doctor if you are breast-feeding.
References
- https://www.drugs.com/mtm/prednisolone.html
- https://books.google.com/books?id=BR9_DQAAQBAJ&pg=PA169
- https://pubchem.ncbi.nlm.nih.gov/compound/prednisolone
- Czock D, Keller F, Rasche FM, Häussler U (2005). "Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids". Clinical Pharmacokinetics. 44 (1): 61–98.
- Sandström, B. "Some observations on the influence of insulin, prednisolone and free amino acids on the glucose metabolism in cultivated liver tissue." Acta Soc Med Ups 71.1(1966): 41-48.
- Gravholt, C. H., et al. "Preferential stimulation of abdominal subcutaneous lipolysis after prednisolone exposure in humans. " Obesity Research 10.8(2012): 774-781.
- Puustinen, T., Punnonen, K., & Jansén, C. T. (1985). Effect of prednisolone on the fatty acid composition and the formation of arachidonate metabolites in human keratinocytes in culture. Prostaglandins Leukotrienes & Medicine, 18(3), 293-299.
- Stichtenoth, D. O., Fauler, J., Zeidler, H., & Frölich, J. C. (1995). Urinary nitrate excretion is increased in patients with rheumatoid arthritis and reduced by prednisolone. Annals of the Rheumatic Diseases, 54(10), 820-824.
- Ost, L., Tydén, G., & Fehrman, I. (1988). Impaired glucose tolerance in cyclosporine-prednisolone-treated renal graft recipients. Transplantation, 46(3), 370-2.
- https://www.rxlist.com/prednisolone-drug.htm#description
- Gristwood, R. W., Llupiá, J., Fernández, A. G., & Berga, P. (1991). Effects of theophylline compared with prednisolone on late phase airway leukocyte infiltration in guinea pigs. International Archives of Allergy & Applied Immunology, 94(1-4), 293-294.
- Akindele, A., Adeneye, A., Olatoye, F., & Benebo, A. (2014). Protective effect of selected calcium channel blockers and prednisolone, a phospholipase-a2 inhibitor, against gentamicin and carbon tetrachloride-induced nephrotoxicity. Human & Experimental Toxicology, 33(8), 831-846.
- B VernonRoberts, J D Jessop, & J Doré. (1973). Effects of gold salts and prednisolone on inflammatory cells. ii. suppression of inflammation and phagocytosis in the rat. Annals of the Rheumatic Diseases, 32(4), 301-307.
- Lee SH (November 2015). "Mechanisms of Glucocorticoid Action in Chronic Rhinosinusitis". Allergy, Asthma & Immunology Research. 7 (6): 534–7.
- Kadmiel, M., & Cidlowski, J. A. (2013). Glucocorticoid receptor signaling in health and disease. Trends in Pharmacological Sciences, 34(9), 518-530.
- A. A. Sandberg and W. R. Slaunwhite. Differences in metabolism of prednisolone-C 14 and cortisol-C TM. J. Clin. Endocrinol. 17:1040-1050 (1957).
- A. Vermeulen. The metabolism of 4-14C prednisolone. J. Endocrinol. 18:278-291 (1959).
- T. Uete and N. Shimano. A simple method for separate determination of delta 4and delta~'4-corticosteroids in urine. Clin. Chem. 17:161-165 (1971).
- J. English, J. Chakraborty, V. Marks, D. J. Trigger, and A. G. Thomson. Prednisolone levels in the plasma and urine: A study of two preparations in man. Br. J. Clin. PharmacoL 2:327-332 (1975).
- R. Leclercq and G. Copinschi. Patterns of plasma levels of prednisolone after oral administration in man. J. Pharmacokin. Biopharm. 2:175-187 (1974).
- P. J. Morrison, I. D. Bradbrook, and H. J. Rogers. Plasma prednisolone levels from enteric and non-enteric coated tablets estimated by an original technique. Br. J. Clin. Pharmacol. 4:597-603 (1977).
- G. P. Lewis, W. J. Jusko, C. W. Burke, and L. Graves. Prednisone side-effects and serum-protein levels. Lancet 2:778-781 (1971).
Description
Prednisolone is the active metabolite of the synthetic corticosteroid prednisone , which is used in the suppression of inflammation and autoimmunity, as well as in other conditions. It alters gene expression through both the glucocorticoid and mineralocorticoid receptors.
Chemical Properties
Crystalline Solid. soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of prednisone in these solvents is approximately 3, 30, and 25 mg/ml, respectively.
Originator
Sterane, Pfizer ,US ,1955
Uses
Prednisolone is used for the same indications as all corticosteroids: rheumatism, polyarthritis, bronchial asthma, neurodermatitis, and eczema.
Definition
ChEBI: Prednisolone is a glucocorticoid that is prednisone in which the oxo group at position 11 has been reduced to the corresponding beta-hydroxy group. It is a drug metabolite of prednisone. It has a role as an adrenergic agent, an anti-inflammatory drug, an antineoplastic agent, an immunosuppressive agent, a drug metabolite, an environmental contaminant and a xenobiotic. It is a glucocorticoid, an 11beta-hydroxy steroid, a 21-hydroxy steroid, a 17alpha-hydroxy steroid, a 20-oxo steroid, a 3-oxo-Delta(1),Delta(4)-steroid, a primary alpha-hydroxy ketone, a tertiary alpha-hydroxy ketone and a C21-steroid. It is functionally related to a Delta(1)-progesterone.
Application
Prednisolone is a synthetic corticosteroid with metabolically interconvertible with prednisone. It mediates its effect by acting on neurokinin 1 receptor. It is used to treat many conditions that cause inflammation, including inflammatory bowel disease (IBD).
brand name
Cortalone(Halsey); Delta-Cortef (Pharmacia & Upjohn); Fernisolone(Ferndale); Meti-Derm (Schering); Prelone (Muro); Prelone(Teva); Sterane (Pfizer).
Therapeutic Function
Glucocorticoid
General Description
Prednisolone,Δ1-hydrocortisone,11β,17,21-trihydroxypregna-1,4-diene-3,20-dione, hasless salt-retention activity than hydrocortisone, but some patients have more frequently experiencedcomplications such as gastric irritation and peptic ulcers.Because of low MC activity, it cannot be used alone for adrenalinsufficiency. Prednisolone is available in varioussalts and esters to maximize its therapeutic utility:
Prednisolone acetate, USP (21-acetate)
Prednisolone sodium phosphate, USP (21-sodiumphosphate)
Prednisolone sodium succinate, USP (21-sodiumsuccinate)
Prednisolone tebutate, USP (21-tebutate).
Hazard
Causes sodium retention; may have side effects similar to cortisone.
Mechanism of action
Prednisolone is hydrocortisone to which has been added a ?1 double bond. This places two double bonds in ring A, which flattens it and increases glucocorticoid action at the expense of mineralocorticoid activity. Prednisolone has fourfold the glucocorticoid activity of hydrocortisone while having approximately half its mineralocorticoid activity. In addition, prednisolone has an increased duration of action compared to hydrocortisone, because the extra double bond in ring A retards its metabolic reduction.
Clinical Use
Prednisolone can be used to treat severe asthmatic attacks that do not respond to conventional treatment, and it is available as the free alcohol for oral administration. The C-21 sodium phosphate (Hydeltrasol) ester is available for parenteral use.
Side effects
A prodrug of prednisolone is prednisone. It is the 11-keto analogue of prednisolone and must be converted in vivo to the active 11β-hydroxy compound, which is necessary to hydrogen bond to Asn-564 in the glucocorticoid receptor. Prednisone should not be used in patients with hepatic dysfunction, because their ability to reduce the 11-keto group with 11β-hydroxysteroid dehydrogenase to the active metabolite may be impaired.
Prednisolone is well tolerated by most people and can start to work very quickly. Side effects can be dependent on the dose and duration of treatment. Short courses of treatment often have fewer side effects than long term use. A Severe allergic reaction is a very rare side effect of prednisolone.
Safety Profile
A poison by intravenous and subcutaneous routes. Moderately toxic by ingestion and intraperitoneal routes. Human teratogenic effects by an unspecified route: developmental abnormalities of the central nervous system; effects on embryo or fetus: fetal death, extra embryonic structures. Human reproductive effects by an unspecified route: stdlbirth. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
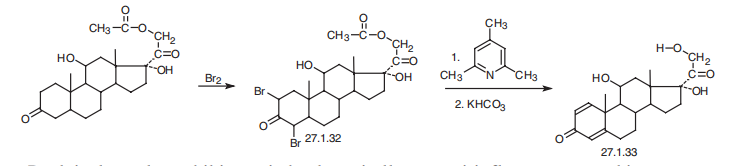
Prednisolone is 11|?,17|á,21-trihydroxypregna-1,4-dien-3,20-dione (27.1.33). Structurally, prednisolone differs from prednisone in that the keto-group at C11 of prednisone is replaced by a hydroxyl group. Prednisolone is synthesized either by microbiological dehydrogenation of C1¨CC2 bond in hydrocortisone [16¨C19], or from 21-acetoxy- 11|?,17|á-dihydroxy-5|á-pregnan-3,20-dione, which undergoes dibromination by molecular bromine in acetic acid at positions C2 and C4, and then the resulting dibromide 27.1.32 is dehydrobrominated by heating it in collidine, which gives prednisolone as an acetate at position C21. Hydrolyzing this compound leads to formation of prednisolone (27.1.33).
Drug interactions
Potentially hazardous interactions with other drugs
Aldesleukin: avoid concomitant use.
Antibacterials: metabolism accelerated by rifamycins
and rifampicin; metabolism possibly inhibited by
erythromycin; concentration of isoniazid possibly
reduced.
Anticoagulants: efficacy of coumarins and
phenindione may be altered.
Antiepileptics: metabolism accelerated by
carbamazepine, fosphenytoin, phenobarbital,
phenytoin and primidone.
Antifungals: increased risk of hypokalaemia with
amphotericin - avoid; metabolism possibly inhibited
by itraconazole and ketoconazole.
Antivirals: concentration possibly increased by
ritonavir.
Ciclosporin: rare reports of convulsions in patients
on ciclosporin and high-dose corticosteroids;
increased levels of prednisolone; increased
ciclosporin levels reported with prednisolone.
Cobicistat: concentration possibly increased by
cobicistat - increased risk of adrenal suppression.
Diuretics: enhanced hypokalaemic effects of
acetazolamide, loop diuretics and thiazide diuretics.
Vaccines: high-dose corticosteroids can impair
immune response to vaccines - avoid with live
vaccines.
Metabolism
Prednisolone is hepatically metabolised and excreted in the urine as sulphate and glucuronide conjugates, with an appreciable proportion of unchanged prednisolone.
Prednisolone Solution Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Baoji Guokang Bio-Technology Co., Ltd. | 0917-3909592 13892490616 | gksales1@gk-bio.com | China | 9310 | 58 |
| Pharmaceuticals Group Corp., Ltd. | +8613004379774 | wu@jiaerke.com | China | 33 | 58 |
| Aurora Industry Co., Ltd. | +86-13591747876 +86-13591747876 | alex1_auco@126.com | China | 299 | 58 |
| Eaglech(shanghai)Pharmaceutical Co., Ltd | +86-2160290089 +86-13884467586 | eagpharm@gmail.com | China | 94 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 | abby@weibangbio.com | China | 8804 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | 1022@dideu.com | China | 3882 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | info@fortunachem.com | China | 5972 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 | Nancy@kangcang.com.cn | China | 341 | 58 |
| Moxin Chemicals | +8617320513646 | mike@molcoo.com | China | 9654 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | sales@capot.com | China | 29792 | 60 |
Related articles
- Understanding the Mechanism, Pharmacokinetics, and Side Effects of Prednisolone
- Prednisolone binds to glucocorticoid receptors, with 70% bioavailability. Metabolism involves prednisone conversion. Overdose ....
- Jan 9,2024
- What is a prednisolone used for?
- Prednisolone is a medicine used to treat a wide range of health problems including allergies, blood disorders, skin diseases, ....
- Dec 23,2022
View Lastest Price from Prednisolone Solution manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-09 | Prednisolone
50-24-8
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2019-08-08 | PREDNISOLONE
52438-85-4
|
US $3.00 / KG | 1KG | 99% | 100kg | Career Henan Chemical Co |
-

- Prednisolone
50-24-8
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- PREDNISOLONE
52438-85-4
- US $3.00 / KG
- 99%
- Career Henan Chemical Co
50-24-8(Prednisolone Solution)Related Search:
1of4