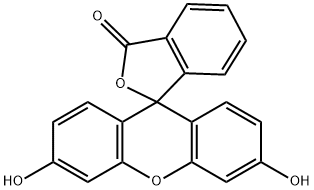
Fluoresceine
- CAS No.
- 2321-07-5
- Chemical Name:
- Fluoresceine
- Synonyms
- SOLVENT YELLOW 94;CI 45350:1;Fluorecein;fluoresceine;KI201;S NO 880;YELLOW 7;CI 45350.1;NSC 667256;angiofluor
- CBNumber:
- CB00130587
- Molecular Formula:
- C20H12O5
- Molecular Weight:
- 332.31
- MDL Number:
- MFCD00005050
- MOL File:
- 2321-07-5.mol
- MSDS File:
- SDS
| Melting point | 320 °C(lit.) |
|---|---|
| Boiling point | 429.44°C (rough estimate) |
| Density | 1.2739 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | room temp |
| solubility | Solubility Insoluble in water, ether, benzene, chloroform; soluble in ethanol, methanol, acetone, Iethyl acetate, N,N-dimethylformamide |
| Colour Index | 45350 |
| form | Powder |
| pka | 2.2, 4.4, 6.7(at 25℃) |
| color | Red to orange |
| PH Range | Pink uorescence (4.0) to green uorescence (6.0) |
| Water Solubility | insoluble |
| λmax | 493.5nm, 496nm, 460nm, 515nm |
| ε(extinction coefficient) |
≥12000 at 282-286nm ≥39000 at 236-240nm ≥70000 at 488-492nm |
| Merck | 14,4159 |
| BRN | 94324 |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Major Application | Organic light-emitting diode, nanoparticles, liquid crystal display, oil products, lip make-up, nucleic acid synthesis, amplification, sequencing and cloning, diagnosis of diabetic retinopathy, detecting yeast, multidrug resistance, proteins, antiHCV antibodies, target genes, enzymatic activity, nerve agent, nucleic acid sequences, imaging lung cancer, prostate cancer |
| Biological Applications | Detecting chromosomal aberration; diagnosing corneal diseases; marking/detecting insects; as antitumor agent; as antihistaminic agent; use in agriculture and plant cultivation; use in cosmetics; use in ophthalmology; as a substrate for measuring α-amylases activity, elastases activity, esterases activity, β-glucuronidases activity, horseradish peroxidases activity, kinases activity, monoamine oxidases (MAOs) activity, phosphatases activity, phospholipases activity, proteases/proteinases activity, telomerases activity activity |
| LogP | 2.980 (est) |
| FDA 21 CFR | 74.1707; 74.2707; 82.1707 |
| CAS DataBase Reference | 2321-07-5(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | TPY09G7XIR |
| ATC code | S01JA01,S01JA51 |
| EPA Substance Registry System | Fluorescein (2321-07-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.47 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 43-36-36/37/38 | |||||||||
| Safety Statements | 22-24/25-37/39-26-36/37/39-27 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LM5075000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 32042000 | |||||||||
| Hazardous Substances Data | 2321-07-5(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Fluoresceine price More Price(41)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 46955 | Fluorescein for fluorescence, free acid | 2321-07-5 | 250mg | $36.4 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46955 | Fluorescein for fluorescence, free acid | 2321-07-5 | 1g | $145 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46955 | Fluorescein for fluorescence, free acid | 2321-07-5 | 100g | $1290 | 2024-03-01 | Buy |
| Sigma-Aldrich | 46955 | Fluorescein for fluorescence, free acid | 2321-07-5 | 500g | $2290 | 2024-03-01 | Buy |
| TCI Chemical | F0095 | Fluorescein | 2321-07-5 | 25g | $20 | 2024-03-01 | Buy |
Fluoresceine Chemical Properties,Uses,Production
Description
Fluorescein is a synthetic organic compound available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications.
Fluorescein is a fluorophore commonly used in microscopy, in a type of dye laser as the gain medium, in forensics and serology to detect latent blood stains, and in dye tracing. Fluorescein has an absorption maximum at 494 nm and emission maximum of 521 nm (in water). The major derivatives are fluorescein isothiocyanate (FITC) and, in oligonucleotide synthesis, 6-FAM phosphoramidite.
Fluorescein also has an isosbestic point (equal absorption for all pH values) at 460 nm. Fluorescein is also known as a color additive (D&C Yellow no. 7). The disodium salt form of fluorescein is known as uranine or D&C Yellow no. 8.
The color of its aqueous solution varies from green to orange as a function of the way it is observed: by reflection or by transmission, as it can be noticed in bubble levels in which fluorescein is added as a colorant to the alcohol filling the tube to increase the visibility of the air bubble and the precision of the instrument. More concentrated solutions of fluorescein can even appear red.
Chemical Properties
Orange-red, crystalline powder. Very dilute alkaline solutions exhibit intense greenishyellow fluorescence by reflected light, while the solution is reddish-orange by transmitted light.soluble in dilute alkalies, boiling alcohol, ether, dilute acids, and
Physical properties
The fluorescence of this molecule is very intense; peak excitation occurs at 494 nm and peak emission at 521 nm.
Fluorescein has a pKa of 6.4, and its ionization equilibrium leads to pH-dependent absorption and emission over the range of 5 to 9. Also, the fluorescence lifetimes of the protonated and deprotonated forms of fluorescein are approximately 3 and 4 ns, which allows for pH determination from non intensity based measurements. The lifetimes can be recovered using time-correlated single photon counting or phase-modulation fluorimetry.
Uses
corneal trama indicator
Uses
A fluorescent tracer used for many applications. It is used in ophthalmology as a tool in the diagnosis of corneal abrasions, corneal ulcers and herpetic corneal infections. It can attach to certain biologically active molecules, thus allowing biologists to target the fluorophore to specific proteins or structures within cells
Uses
Fluorescein is used as a fluorescent tracer for many applications including in a type of dye laser as the gain medium, in forensics and serology to detect latent blood stains and in dye tracing. It is used to localise multiple muscular ventricular septal defects during open heart surgery and confirm the presence of any residual defects. It is applied to teeth to reveal plaque.
Application
Biochemical research
In cellular biology, the isothiocyanate derivative of fluorescein is often used to label and track cells in fluorescence microscopy applications (for example, flow cytometry). Additional biologically active molecules (such as antibodies) may also be attached to fluorescein, allowing biologists to target the fluorophore to specific proteins or structures within cells. This application is common in yeast display.
Health care applications
"Fluorescein sodium", the sodium salt of fluorescein, is used extensively as a diagnostic tool in the field of ophthalmology and optometry, where topical fluorescein is used in the diagnosis of corneal abrasions, corneal ulcers and herpetic corneal infections. It is also used in rigid gas permeable contact lens fitting to evaluate the tear layer under the lens.
Uses in river systems
In 1966, environmentalists forced a change to a vegetable-based dye to protect local wildlife. Other uses of fluorescein include using it as a water-soluble dye added to rainwater in environmental testing simulations to aid in locating and analyzing any water leaks, and in Australia and New Zealand as a methylated spirit dye.
Oil field application
Fluorescein dye solutions, typically 15 % active, are commonly used as an aid to leak detection during hydrostatic testing of sub sea oil and gas pipelines and other subsea infrastructure. Leaks can be detected by divers carrying ultraviolet lights.
Definition
A fluorescent dye used as an absorption indicator.
Definition
ChEBI: A xanthene dye that is highly fluorescent, detectable even when present in minute quantities. Used forensically to detect traces of blood, in analytical chemistry as an indicator in silver nitrate titrations and in microscopy.
General Description
Yellow amorphous solid or orange-red crystals. Latter have greenish-yellow fluorescence by reflected light. Insoluble in water. Soluble in dilute aqueous bases. Very dilute alkaline solutions exhibit intense, greenish-yellow fluorescence by reflected light. Low toxicity. May be sensitive to prolonged exposure to light.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Fluorescein is incompatible with strong oxidizers. Also incompatible with acids, acid salts and salts of heavy metals. .
Fire Hazard
Flash point data for Fluorescein is not available, but Fluorescein is probably combustible.
Safety Profile
Poison by intravenous route. Moderately toxic by intraperitoneal route. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. See also FLUORESCEIN SODIUM.
Safety
Topical, oral, and intravenous use of fluorescein can cause adverse reactions, including nausea, vomiting, hives, acute hypotension, anaphylaxis and related anaphylactoid reaction, causing cardiac arrest and sudden death due to anaphylactic shock.
The most common adverse reaction is nausea, due to a difference in the pH from the body and the pH of the sodium fluorescein dye; a number of other factors , however, are considered contributors as well. The nausea usually is transient and subsides quickly. Hives can range from a minor annoyance to severe, and a single dose of antihistamine may give complete relief. Anaphylactic shock and subsequent cardiac arrest and sudden death are very rare, but because they occur within minutes, a health care provider who uses fluorescein should be prepared to perform emergency resuscitation.
Synthesis
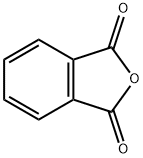
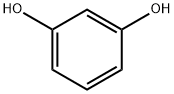
Fluorescein was first synthesized by Adolf von Baeyer in 1871. It can be prepared from phthalic anhydride and resorcinol in the presence of zinc chloride via the Friedel - Crafts reaction.
A second method to prepare fluorescein uses methanesulfonic acid as a Br?nsted acid catalyst. This route has a high yield under milder conditions.
Derivatives
There are many fluorescein derivatives. For example, fluorescein isothiocyanate 1, often abbreviated as FITC, is the original fluorescein molecule functionalized with an isothiocyanate group ( - N = C = S ) , replacing a hydrogen atom on the bottom ring of the structure. This derivative is reactive towards primary amine groups of biologically relevant compounds including intracellular proteins to form a thiourea linkage. A succinimidyl ester functional group attached to the fluorescein core, creating NHS-fluorescein, forms another common amine-reactive derivative, yielding more stable amide adducts. Penta fluoro phenyl esters (PFP) and tetra fluoro phenyl esters (TFP) are other useful reagents. In oligonucleotide synthesis, several phosphoramidite reagents containing protected fluorescein, e.g. 6-FAM phosphoramidite 2, are widely used for the preparation of fluorescein-labeled oligonucleotides.
Other green dyes include Oregon Green, Tokyo Green, SNAFL, and carboxy naphtho fluorescein. These dyes, along with newer fluoro phores such as Alexa 488, Fluo Probes 488 and DyLight 488, have been tailored for various chemical and biological applications where higher photo stability, different spectral characteristics, or different attachment groups are needed.
Fluoresceine Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 | sales@simchoicechem.com | China | 254 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | sales1@chuanghaibio.com | China | 5868 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 20234 | 58 |
| Hebei Saisier Technology Co., LTD | +86-18400010335 +86-18034520335 | admin@hbsaisier.cn | China | 1015 | 58 |
| Hebei Longbang Technology Co., LTD | +86-18633929156 +86-18633929156 | admin@hblongbang.com | China | 972 | 58 |
| HebeiShuoshengImportandExportco.,Ltd | +86-18532138899 +86-18532138899 | L18532138899@163.com | China | 939 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | niki@zlchemi.com | China | 7245 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-+86-371-66670886 | info@dakenam.com | China | 19805 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21628 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
Related articles
- Fluorescein: A Versatile Compound in Chemistry
- Fluorescein is extensively used compound in the field of chemistry, known for its intense fluorescence and versatility across ....
- Sep 10,2024
View Lastest Price from Fluoresceine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-08-21 | Sodium tetraborate pentahydrate
12179-04-3
|
1kg | 99% | 100tons | Anhui Ruihan Technology Co., Ltd |
-

- Sodium tetraborate pentahydrate
12179-04-3
- 99%
- Anhui Ruihan Technology Co., Ltd