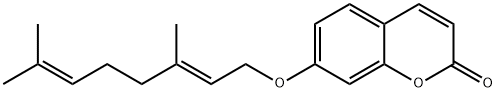
AURAPTENE
- CAS No.
- 495-02-3
- Chemical Name:
- AURAPTENE
- Synonyms
- aurapten;AURAPTENE;Aurapten -RM;Aurapten【C19H22O3】;AURAPTENE(RG) 25mg;7-GERANYLOXYCOUMARIN;Auraptene, 10 mM in DMSO;Auraptene, Activates PPARalpha and PPARgamma;Auraptene, 98%, from Citrus maxima (Burm.) Merr.;(E)-7-((3,7-Dimethylocta-2,6-dien-1-yl)oxy)-2H-chromen-2-one
- CBNumber:
- CB3269606
- Molecular Formula:
- C19H22O3
- Molecular Weight:
- 298.38
- MDL Number:
- MFCD00075948
- MOL File:
- 495-02-3.mol
- MSDS File:
- SDS
| Melting point | 91 °C |
|---|---|
| Boiling point | 455.5±45.0 °C(Predicted) |
| Density | 1.079±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO: >20mg/mL |
| form | powder |
| color | white to off-white |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| LogP | 5.690 (est) |
| FDA UNII | F79I1ZEL2E |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P280-P305+P351+P338 |
| WGK Germany | 3 |
| HS Code | 2932990090 |
AURAPTENE price More Price(35)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | A9861 | Auraptene ≥98% (HPLC) | 495-02-3 | 5mg | $118 | 2024-03-01 | Buy |
| Sigma-Aldrich | A9861 | Auraptene ≥98% (HPLC) | 495-02-3 | 25mg | $372 | 2024-03-01 | Buy |
| TCI Chemical | A3147 | Auraptene | 495-02-3 | 25MG | $112 | 2024-03-01 | Buy |
| TCI Chemical | A3147 | Auraptene | 495-02-3 | 100MG | $305 | 2024-03-01 | Buy |
| Cayman Chemical | 14000 | Auraptene ≥98% | 495-02-3 | 5mg | $29 | 2024-03-01 | Buy |
AURAPTENE Chemical Properties,Uses,Production
Description
Auraptene (495-02-3) is a bioactive terpenoid occurring in a variety of citrus fruits and possessing therapeutic potential.1?? Displays neuritogenic activity2?and neuroprotective effects via suppression of inflammation and induction of GDNF and BDNF in neuronal cells3. Attenuates ROS production and enhances mitochondrial respiration which mitigates Parkinson’s disease-like behavior.4?Displays hepatoprotective5?and chemopreventive activity6.
Uses
antineoplastic, apoptosis inducer
Uses
Auraptene is a natural bioactive monoterpene coumarin ether. Auraptene has shown a remarkable effect in the prevention of degenerative diseases.
Definition
ChEBI: Auraptene is a member of the class of coumarins that is umbelliferone in which the phenolic hydrogen has been replaced by a geranyl group. Ii is isolated from several edible fruits and vegetables and exhibits a variety of therapeutic properties. It has a role as a plant metabolite, an antineoplastic agent, an apoptosis inducer, a dopaminergic agent, a neuroprotective agent, an antihypertensive agent, a gamma-secretase modulator, a vulnerary, an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, a PPARalpha agonist, a gastrointestinal drug, a matrix metalloproteinase inhibitor, an antioxidant and a hepatoprotective agent. It is a member of coumarins and a monoterpenoid. It is functionally related to an umbelliferone.
in vivo
Auraptene (200, 500 ppm, mixed in the diet, p.o.) delays the tumor progression of breast cancer rats by inhibiting cyclin D1 protein[3]. Auraptene (100, 500 ppm, mixed in the diet, p.o.) alleviates gastritis by reducing Helicobacter pylori colonization and pro-inflammatory mediator production in C57BL/6 mice[4]. Auraptene (5, 50 mg/kg, 6 weeks, p.o.) prevents heart failure caused by myocardial infarction by activating peroxisome proliferator activated receptor alpha (PPAR alpha) in rats [5]. Auraptene (2, 4, 8, 16 mg/kg, 5 weeks, p.o.) exhibits anti hypertensive effects in hypertensive rats by reducing mean systolic blood pressure[8].
| Animal Model: | Mammary carcinogenesis model in female Sprague Dawley rats[3]. |
| Dosage: | 200, 500 ppm |
| Administration: | Oral gavage (p.o.); mixed in the diet |
| Result: | Delayed median time to tumor by 39 days and reduced Insulin like Growth Factor-1 (IGF-1, 10 ng/mL)-induced cyclin D1 expression by 40% in MCF-7 cells. |
| Animal Model: | Female C57BL/6 mice[4]. |
| Dosage: | 100, 500 ppm |
| Administration: | Oral gavage (p.o.); mixed in the diet |
| Result: | Inhibited H. pylori–induced expression and/or production of CD74, macrophage migration inhibitory factor, interleukin-1b, and tumor necrosis factor-a in gastric mucosa, together with serum macrophage inhibitory protein-2. |
| Animal Model: | Sprague–Dawley rats with moderate myocardial infarction[5]. |
| Dosage: | 5, 50 mg/kg |
| Administration: | Oral gavage (p.o.); 6 weeks |
| Result: | Suppresses PE-induced hypertrophic responses in cardiomyocytes. Prevented the development of cardiac hypertrophy and fibrosis in rats with myocardial infarction. |
| Animal Model: | Desoxycorticosterone acetate (DOCA) salt induced hypertensive rats[8]. |
| Dosage: | 2, 4, 8, 16 mg/kg |
| Administration: | Oral gavage (p.o.); 5 weeks |
| Result: | Reduced the mean systolic blood pressure (MSBP) in DOCA salt treated rats. |
IC 50
MMP-2
References
1) Bibak et al. (2019), A Review of the Pharmacological and Therapeutic Effects of Auraptene; Biofactors, 45 867 2) Furukawa et al. (2012), Neurotrophic Effects of Citrus Auraptene: Neuritogenic Activity in PC12 Cells; Int. J. Mol. Sci., 13 5338 3) Furukawa et al. (2020), Citrus Auraptene Induces Expression of Brain-Derived Neurotrophic Factor in Neuro2a Cells; Molecules, 25 1117 4) Jang et al. (2019), Auraptene Mitigates Parkinson’s Disease-Like Behavior by Protecting Inhibition of Mitochondrial Respiration and Scavenging Reactive Oxygen Species; Int. J. Mol. Sci., 20 3409 5) Wang et al. (2019), Hepatoprotection of Auraptene From the Peels of Citrus Fruits Against 17α-ethinylestradiol-induced Cholestasis in Mice by Activating Farnesoid X Receptor; Food Funct., 10 3839 6) Tanaka et al. (1998), Citrus Auraptene Exerts Dose-Dependent Chemopreventative Activity in Rat Large Bowel Tumorigenesis: The Inhibition Correlates With Suppression of Cell Proliferation and Lipid Peroxidation and With Induction of Phase II Drug-Metabolizing Enzymes; Cancer Res., 58 2550
AURAPTENE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 | zheyansh@163.com | CHINA | 3619 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | scglp@glp-china.com | CHINA | 1824 | 58 |
| Chengdu Biopurify Phytochemicals Ltd. | +86-28-82633860; +8618080483897 | sales@biopurify.com | China | 3772 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22963 | 58 |
| Shanghai Standard Technology Co., Ltd. | 18502101150 | ft-sales@nature-standard.com | CHINA | 1923 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | factory@coreychem.com | China | 29808 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569262 +86-15003564040 | 1087@dideu.com | China | 3270 | 58 |
| Wuhan ChemNorm Biotech Co.,Ltd. | +86-27-8439 4403 18971486879 | sales@chemnorm.com | CHINA | 2935 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 32159 | 58 |
View Lastest Price from AURAPTENE manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2025-01-03 | Auraptene
495-02-3
|
US $0.00-0.00 / KG | 5mg | 99%HPLC | 2000tons | Shaanxi Dideu Medichem Co. Ltd | |
 |
2024-11-19 | Auraptene
495-02-3
|
US $30.00-59.00 / mg | 99.17% | 10g | TargetMol Chemicals Inc. | ||
 |
2023-02-24 | Auraptene
495-02-3
|
US $0.00 / mg | 20mg | ≥98%(HPLC) | 100 g | Shanghai Standard Technology Co., Ltd. |