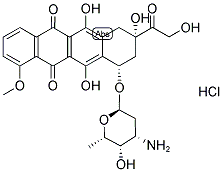
Doxorubicin hydrochloride
- CAS No.
- 25316-40-9
- Chemical Name:
- Doxorubicin hydrochloride
- Synonyms
- DOX;DOXORUBICIN HCL;ADR;Doxorubicin HCI;CAELYX;DOX HCl;DOX HYDROCHLORIDE;ADRIAMYCIN HYDROCHLORIDE;(8s-cis)-10-[(3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxynaphthacene-5,12-dione hydrochloride;(8S,10S)-10-[[(2R,4S,5S,6S)-4-Amino-5-hydroxy-6-methyl-2-tetrahydropyranyl]oxy]-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione Hydrochloride
- CBNumber:
- CB0110633
- Molecular Formula:
- C27H30ClNO11
- Molecular Weight:
- 579.98
- MOL File:
- 25316-40-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/4 21:45:01
| Melting point | 216 °C (dec.)(lit.) |
|---|---|
| alpha | D20 +248±2° (c = 0.1 in methanol) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO at 100 mg/ml; very poorly soluble in ethanol; soluble in water at 10 mg/ml with slight warming. |
| pka | pKa 8.25±0.60 (Uncertain);8.43±0.70 (Uncertain);11.9±0.4 (Uncertain);12.95±0.1 (Uncertain);13.8±0.70 (Uncertain) |
| form | solid |
| color | orange to dark red |
| Water Solubility | Soluble in water. |
| λmax | 497nm(H2O)(lit.) |
| Merck | 14,3439 |
| BRN | 4229251 |
| Stability | Stable for 1 year from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 3 months. |
| InChIKey | MWWSFMDVAYGXBV-RUELKSSGSA-N |
| CAS DataBase Reference | 25316-40-9(CAS DataBase Reference) |
| EPA Substance Registry System | Doxorubicin hydrochloride (25316-40-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H340-H350-H360FD | |||||||||
| Precautionary statements | P201-P202-P264-P270-P301+P312-P308+P313 | |||||||||
| Hazard Codes | T,T+,Xi | |||||||||
| Risk Statements | 45-22-40-26/27/28-36/38 | |||||||||
| Safety Statements | 53-45-36/37/39-22-7/9 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | QI9295900 | |||||||||
| F | 10-21 | |||||||||
| HS Code | 29419090 | |||||||||
| Toxicity | LD50 i.v. in mice: 21.1 mg/kg (Bertazzoli, 1970) | |||||||||
| NFPA 704 |
|
Doxorubicin hydrochloride price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1789 | Doxorubicin Hydrochloride Pharmaceutical Secondary Standard; Certified Reference Material | 25316-40-9 | 200MG | ₹39846.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 44583 | Doxorubicin hydrochloride suitable for fluorescence, 98.0-102.0% (HPLC) | 25316-40-9 | 1MG | ₹5683.13 | 2022-06-14 | Buy |
| TCI Chemicals (India) | D4193 | Doxorubicin Hydrochloride | 25316-40-9 | 25MG | ₹9000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | D4193 | Doxorubicin Hydrochloride | 25316-40-9 | 100MG | ₹17400 | 2022-05-26 | Buy |
| ottokemi | D 7175 | Doxorubicin hydrochloride 98.0-102% | 25316-40-9 | 1mg | ₹4491 | 2022-05-26 | Buy |
Doxorubicin hydrochloride Chemical Properties,Uses,Production
Chemical Properties
Doxorubicin is an orange to red cake-like or needle-like crystalline solid. It is a cytotoxic anthracycline antibiotic isolated from cultures of Streptomyces peucetius var. caesius. Doxorubicin hydrochloride is an orange-red, crystalline, hygroscopic powder that is soluble in water and slightly soluble in methanol.
Uses
Doxorubicin hydrochloride (adriamycin hydrochloride) is an antitumour agent that has been formulated as a salt to achieve higher water solubility. While the salt shares the same pharmacological properties as doxorubicin free base, its greater water solubility may offer advantages in some in vitro applications. Physicochemical properties and chromatographic behaviour will depend on whether the pH is buffered. In non-pH controlled systems the free base and salt may behave differently.
Biological Functions
The C13 substituent of doxorubicin is hydroxymethyl, which retards the action of cytosolic aldoketoreductase and slows the conversion to the equally active, but chronically cardiotoxic, doxorubicinol.
General Description
Adriamycin hydrochloride appears as orange-red thin needles. Aqueous solutions yellow-orange at acid pHs, orange-red at neutral pHs, and violet blue over pH 9. Doxorubicin is available as both the conventional dosageform and a liposomal preparation, both of which are administeredby infusion. Doxorubicin HCl powder is available in10-, 20-, 50-, and 150-mg vials and is widely used in treatingvarious cancers, including leukemias, soft and bone tissuesarcomas, Wilms tumor, neuroblastoma, small cell lungcancer, and ovarian and testicular cancer.
Air & Water Reactions
Water soluble.
Reactivity Profile
Amines, like Doxorubicin hydrochloride, are weak chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard
Doxorubicin hydrochloride is probably combustible.
Biological Activity
Antitumor antibiotic agent that inhibits DNA topoisomerase II. DNA intercalator that inhibits nucleic acid synthesis and induces apoptosis.
Mechanism of action
Liposomes are taken up selectively into tumor cells, presumably because of their persistence in the bloodstream and enhanced permeability of tumor vascular membranes. In liposomal form, the drug is protected against enzymes that generate cardiotoxic free radicals, although this form of the drug can still induce potentially fatal congestive heart failure. Clinical experience with the liposomal formulation is limited, and few studies comparing the long-term toxicity with that of conventional doxorubicin therapy have been conducted. Therefore, all precautions outlined for the use of doxorubin also are employed when the liposomal formulation is used.
Clinical Use
Doxorubicin is utilized either alone or in combination therapy to treat a wide range of neoplastic disorders, including hematologic cancers and solid tumors in breast, ovary, stomach, bladder, and thyroid gland. A liposomal formulation of doxorubicin is used in the treatment of AIDS-related Kaposi's sarcoma and organoplatinum-resistant ovarian cancer.
Potential Exposure
An antibiotic product from streptomyces, used as anticancer drug
Metabolism
This contributes to the longer duration of action compared to analogues that have CH3 at this position (e.g., daunorubicin). Doxorubicin is highly lipophilic and concentrates in the liver, lymph nodes, muscle, bone marrow, fat, and skin. Elimination is triphasic, and the drug has a terminal half-life of 30 to 40 hours. The majority of an administered dose is excreted in the feces.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Doxorubicin hydrochloride Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLP Pharma Standards | +91 9866074638 | Hyderabad, India | 1644 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Sakar Healthcare | +91-8976292690 +91-9967572302 | Gujarat, India | 47 | 58 | Inquiry |
| BDR Pharmaceuticals International Pvt Ltd | +91-2240560560 +91-7718884418 | Maharashtra, India | 206 | 58 | Inquiry |
| Vyshno Bio Sciences | 91-7095439993 | Hyderabad, India | 239 | 58 | Inquiry |
| Trichem Healthcare Pvt. Ltd. | 91-22-66083333 | Maharashtra, India | 9 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Rawia International HealthCare Pvt. Ltd. | 91-22-26425001 | Maharashtra, India | 170 | 58 | Inquiry |
| Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group) | 91-22-42361111 | Maharashtra, India | 147 | 58 | Inquiry |
| Pharmaffiliates Analytics & Synthetics (P) Ltd. | 91-172-5066494 | Haryana, India | 631 | 58 | Inquiry |
Related articles
- Doxorubicin Hydrochloride: Anticancer Mechanisms and Highly Sensitive Determination
- Doxorubicin hydrochloride exhibits potent anticancer effects via DNA interaction and immune enhancement, while a new nanocompo....
- Jul 4,2024
- Doxorubicin Hydrochloride Injection
- Doxorubicin Hydrochloride Injection, USP, has been used successfully to produce regression in disseminated neoplastic conditio....
- Jun 28,2024
- Adverse reactions of doxorubicin hydrochloride
- Doxorubicin hydrochloride is an anti-tumor antibiotic.
- Mar 30,2022
25316-40-9(Doxorubicin hydrochloride)Related Search:
1of4
chevron_right




