Magnesium dioxide
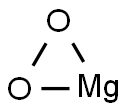
- CAS No.
- 14452-57-4
- Chemical Name:
- Magnesium dioxide
- Synonyms
- 24-28%asMgO2;Magnesiumdioxid;Magnesium dioxide;MAGNESII PEROXIDUM;Magnesium peroxidum;foranalyticalpurpose;MAGNESIUM PEROXIDE LIGHT;magnesiumperoxide(mg(o2));Magnesium peroxide light, Ph Eur;MAGNESIUM PEROXIDE LIGHT, PH HELV
- CBNumber:
- CB0117465
- Molecular Formula:
- MgO2
- Molecular Weight:
- 56.3
- MOL File:
- 14452-57-4.mol
- Modify Date:
- 2024/5/28 19:59:05
| solubility | Practically insoluble in water and in ethanol (96 per cent). It dissolves in dilute mineral acids. |
|---|---|
| Merck | 13,5704 |
| LogP | -0.425 (est) |
| CAS DataBase Reference | 14452-57-4 |
| EPA Substance Registry System | Magnesium peroxide (Mg(O2)) (14452-57-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H272-H314 |
| Precautionary statements | P220-P280-P305+P351+P338-P310 |
| Hazard Codes | O,C |
| Risk Statements | 8-34 |
| Safety Statements | 17-36-45-36/37/39-27-26 |
| RIDADR | UN 1476 5.1/PG 2 |
| WGK Germany | 3 |
Magnesium dioxide Chemical Properties,Uses,Production
Chemical Properties
White or slightly yellow, amorphous, light powder.
Uses
Bleaching and oxidizing agent, medicine (antacid).
General Description
A white powder. Noncombustible but accelerates the burning of combustible material, if the combustible material is finely divided the mixture may be explosive. Mixtures of combustible material and the peroxide can be ignited by friction or contact with moisture. Used in medicine, and as a bleaching agent.
Air & Water Reactions
Insoluble in water and slowly decomposed by water, liberating oxygen.
Reactivity Profile
Mixtures of combustible material and the peroxide can be ignited with friction or moisture [AAR 1991]. Water gradually decomposes Magnesium dioxide liberating oxygen, with dilute acids Magnesium dioxide forms hydrogen peroxide, when strongly heated Magnesium dioxide losses all peroxide oxygen [Merck 11th ed. 1989].
Hazard
Powerful oxidizer and dangerous fire risk, reacts with acidic materials and moisture.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
A powerful oxidizer. Probably a severe irritant to the eyes, skin, and mucous membranes. Flammable by chemical reaction with acidic materials and moisture; an oxidizing agent. Dangerous; reacts vigorously with reducing agents; will decompose violently in or near a fire. See also MAGNESIUM COMPOUNDS and PEROXIDES, INORGANIC.
Potential Exposure
Magnesium peroxide is used as a bleaching and oxidizing agent, and in the manufacture of antacids and antiinfective drugs.
Shipping
UN1476 Magnesium peroxide, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
Powerful oxidizer. Dangerous fire risk with flammable and combustible materials. Violent reaction with acids. Keep away from moisture; causes the release of oxygen and heat.
Magnesium dioxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Suvidhi Industries | 08048981309 | Gujarat, India | 39 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8822 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24738 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | China | 35426 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |
| Hubei Enxing Biotechnology Co., Ltd | 16621771607 | China | 8261 | 58 | Inquiry |
| Shaanxi Xihua Chemical Industry Co., Ltd | 17691182729 15529505138 | China | 9963 | 58 | Inquiry |
| Henan Dobe Chemical Co. , Ltd. | 0370-6999054 17334723986 | China | 1329 | 58 | Inquiry |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 | China | 20807 | 60 | Inquiry |
14452-57-4(Magnesium dioxide)Related Search:
1of4
chevron_right




