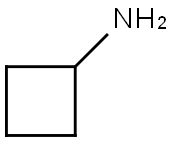
Cyclobutylamine
- CAS No.
- 2516-34-9
- Chemical Name:
- Cyclobutylamine
- Synonyms
- Cyclobutanamine;AMINOCYCLOBUTANE;obutyL;AURORA KA-7608;CYCLOBUTYLAMINE;Ring-butylaMine;1-Cyclobutanamine;CyclobutylaMine 98%;Cyclobutylamine,98%;Cyclobutane-1-amine
- CBNumber:
- CB0134657
- Molecular Formula:
- C4H9N
- Molecular Weight:
- 71.12
- MOL File:
- 2516-34-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/8 18:06:34
| Melting point | 133-135.5 °C(Solv: ligroine (8032-32-4); dichloromethane (75-09-2)) |
|---|---|
| Boiling point | 81.5 °C752 mm Hg(lit.) |
| Density | 0.833 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 24 °F |
| storage temp. | 2-8°C |
| pka | 10.80±0.20(Predicted) |
| form | Liquid |
| color | Clear colorless to light yellow |
| Water Solubility | slightly soluble |
| Sensitive | Hygroscopic |
| BRN | 2069297 |
| InChIKey | KZZKOVLJUKWSKX-UHFFFAOYSA-N |
| CAS DataBase Reference | 2516-34-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Cyclobutylamine(2516-34-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H314 | |||||||||
| Precautionary statements | P210-P233-P240-P280-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | F,C,Xi | |||||||||
| Risk Statements | 11-34-20/21/22-36/37/38 | |||||||||
| Safety Statements | 16-26-36/37/39-45-33-7/9 | |||||||||
| RIDADR | UN 2733 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29213000 | |||||||||
| NFPA 704 |
|
Cyclobutylamine price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 225185 | Cyclobutylamine 98% | 2516-34-9 | 1G | ₹4481.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 225185 | Cyclobutylamine 98% | 2516-34-9 | 5G | ₹22256.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 225185 | Cyclobutylamine 98% | 2516-34-9 | 25G | ₹75201.28 | 2022-06-14 | Buy |
| ALFA India | ALF-A13423-14 | Cyclobutylamine, 98% | 2516-34-9 | 25g | ₹69543 | 2022-05-26 | Buy |
| ALFA India | ALF-A13423-06 | Cyclobutylamine, 98% | 2516-34-9 | 5g | ₹13300 | 2022-05-26 | Buy |
Cyclobutylamine Chemical Properties,Uses,Production
Chemical Properties
Clear colorless to light yellow liquid
Uses
Cyclobutylamine is a substrate employed in a microwave-assisted synthesis of 7-azaindoles from dihalopyridines. Also it is a reactant in the preparation of imidazo[1,2-b]pyridazine derivatives as selective and orally available Mps1 (TTK) kinase inhibitors.
Preparation
The preparation of cyclobutylamine from cyclobutanecarboxylic acid and hydrazoic acid has been reported previously. Cyclobutylamine has also been prepared by the Hofmann-type rearrangement of cyclobutanecarboxamide. More recently it has been prepared in 82–87% overall yield from cyclobutanecarboxamide by oxidative rearrangement with lead tetraacetate or iodosobenzene diacetate.
Synthesis of cyclobutylamine
Purification Methods
It has been purified by steam distillation. The aqueous distillate (e.g. 2L) is acidified with 3N HCl (90mL) and evaporated to dryness in a vacuum. The hydrochloride is treated with a few mL of H2O, cooled in ice and a slush of KOH pellets ground in a little H2O is added slowly in portions and keeping the solution very cold. The amine separates as an oil from the strongly alkaline solution. The oil is collected, dried over solid KOH and distilled using a vacuum jacketed Vigreux column (p 11) and protected from CO2 using a soda lime tube. The fraction boiling at 79-83o is collected, dried over solid KOH for 2days and redistilled over a few pellets of KOH (b 80.5-81.5o). Best distil in a dry N2 atmosphere. The purity can be checked by GLC using a polyethylene glycol on Teflon column at 72o, 15 psi, flow rate of 102 mL/min of He. The sample can appear homogeneous but because of tailing it is not possible to tell if H2O is present. The NMR in CCl4 should show no signals less than 1 ppm from TMS. The hydrochloride has a multiplet at ca 1.5-2.6ppm (H 2,2,4,3,3,4,4), a quintet at 3.8 ppm (H 1) and a singlet at 4.75 for NH2 [Roberts & Chambers J Am Chem Soc 73 2509 1951]. The benzenesulfonamide has m 85-86o (from aqueous MeOH) and the benzoyl derivative has m 120.6-121.6o. [Roberts & Mazur J Am Chem Soc 73 2509 1951, Iffland et al. J Am Chem Soc 75 4044 1953, Werner & Casanova Jr Org Synth Coll Vol V 273 1973, Beilstein 12 IV 3.]
Cyclobutylamine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1269 | 58 | Inquiry |
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| BTC pharm India | +918790379245 | Telangana, India | 145 | 58 | Inquiry |
| ALLCHEM LIFESCIENCE PVT. LTD | +91 957 472 2211 | New Delhi, India | 322 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| A. B. Enterprises | +91-8048077585 | Mumbai, India | 1396 | 58 | Inquiry |
| BTC Nantong Pharmaceuticals Technology Co.,Ltd | +8618068193575 | China | 254 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| BTC pharm India | 58 |
| GLR Innovations | 58 |
| BTC pharm India | 58 |
| ALLCHEM LIFESCIENCE PVT. LTD | 58 |
| A.J Chemicals | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| A. B. Enterprises | 58 |
| BTC Nantong Pharmaceuticals Technology Co.,Ltd | 58 |





