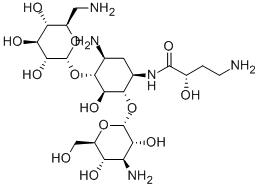
AMIKACIN
- CAS No.
- 37517-28-5
- Chemical Name:
- AMIKACIN
- Synonyms
- AMK;amikacin hydrate;Amikacin base;amikin;amicacin;Amikacine;Amikacinum;Amikacin CRS;AndraMine-d12;(s)-y
- CBNumber:
- CB8146049
- Molecular Formula:
- C22H43N5O13
- Molecular Weight:
- 585.6
- MOL File:
- 37517-28-5.mol
- Modify Date:
- 2024/8/28 13:53:27
| Melting point | 203℃ |
|---|---|
| alpha | D23 +99° (c = 1.0 in water) |
| Boiling point | 642.23°C (rough estimate) |
| Density | 1.3764 (rough estimate) |
| refractive index | 1.7500 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| pka | pKa 8.1 (Uncertain) |
| form | solid |
| color | white to off-white |
| optical activity | 99(c 1.0) |
| Water Solubility | Soluble in water (partly). |
| Merck | 13,404 |
| BRN | 1445422 |
| Stability | Hygroscopic |
| EPA Substance Registry System | Amikacin (37517-28-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H317 | |||||||||
| Precautionary statements | P280 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-24/25 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | WK1955000 | |||||||||
| F | 10-34 | |||||||||
| HS Code | 29419090 | |||||||||
| Toxicity | LD50 in mice of solns pH 6.6, pH 7.4 (mg/kg): 340, 560 i.v. (Kawaguchi) | |||||||||
| NFPA 704 |
|
AMIKACIN price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | PHR1654 | Amikacin Pharmaceutical Secondary Standard; Certified Reference Material | 37517-28-5 | 1G | ₹14321.48 | 2022-06-14 | Buy |
| SRL | 81396 | Amikacin (AMK) free base, 98% | 37517-28-5 | 50mg | ₹2330 | 2022-05-26 | Buy |
| SRL | 81396 | Amikacin (AMK) free base, 98% | 37517-28-5 | 1Gms | ₹5240 | 2022-05-26 | Buy |
| SRL | 81396 | Amikacin (AMK) free base, 98% | 37517-28-5 | 5Gms | ₹14540 | 2022-05-26 | Buy |
| SRL | 81396 | Amikacin (AMK) free base, 98% | 37517-28-5 | 25Gms | ₹55820 | 2022-05-26 | Buy |
AMIKACIN Chemical Properties,Uses,Production
Description
Amikacin is made semisynthetically from kanamycin A. Interestingly, the L-hydroxyaminobutyryl amide (HABA) moiety attached to N-3 inhibits adenylation and phosphorylation in the distant amino sugar ring (at C-2′and C-3′), even though the HABA substituent is not where the enzymatic reaction takes place. This effect is attributed to decreased binding to the R factor–mediated enzymes.
Chemical Properties
white crystalline powder
Uses
Amikacin is a semi-synthetic derivative of kanamycin. It is much less sensitive to the enzymes that inactivate aminoglycoside antibiotics. The spectrum is similar to that of gentamicin. Amikacin principally finds use in the treatment of infections arising from bacteria that are resistant to gentamicin and/or tobramycin.
Definition
ChEBI: An amino cyclitol glycoside that is kanamycin A acylated at the N-1 position by a 4-amino-2-hydroxybutyryl group.
Antimicrobial activity
Among other organisms, Acinetobacter,
Alkaligenes, Campylobacter, Citrobacter, Hafnia, Legionella,
Pasteurella, Providencia, Serratia and Yersinia spp. are usually
susceptible in vitro. Stenotrophomonas maltophilia, many nonaeruginosa
pseudomonads and Flavobacterium spp. are resistant.
M. tuberculosis (including most streptomycin-resistant
strains) and some other mycobacteria (including M. fortuitum
and the M. avium complex) are susceptible; most other mycobacteria,
including M. kansasii, are resistant. Nocardia asteroides
is susceptible.
It exhibits typical aminoglycoside characteristics, including
an effect of divalent cations on its activity against Ps. aeruginosa
analogous to that seen with gentamicin and synergy with
β-lactam antibiotics.
Acquired resistance
Amikacin is unaffected by many of the modifying enzymes
that inactivate gentamicin and tobramycin and is consequently active against staphylococci,
enterobacteria and Pseudomonas that owe their resistance
to the production of those enzymes. However, AAC(6′),
ANT(4′) and some forms of APH(3′) can confer resistance;
because these enzymes generally do not confer gentamicin
resistance, amikacin-resistant strains can be missed in routine
susceptibility tests when gentamicin is used as the representative
aminoglycoside.
There have been reports of resistance arising during treatment
of infections due to Serratia spp. and Ps. aeruginosa.
Outbreaks of infection with multiresistant strains of enterobacteria
and Ps. aeruginosa have occurred after extensive use,
particularly in burns units. Bacteria that owe their resistance
to the expression of ANT(4′) have been described in Staph.
aureus, coagulase-negative staphylococci, Esch. coli, Klebsiella
spp. and Ps. aeruginosa. In E. faecalis, resistance to penicillin–
aminoglycoside synergy has been associated with plasmidmediated
APH(3′). Resistance in Gram-negative organisms is
usually caused by either reduced accumulation of the drug or,
more commonly, by the aminoglycoside-modifying enzymes
AAC(6′) or AAC(3)-VI. The latter enzyme is usually found in
Acinetobacter spp., but has also been found, encoded by a transposon,
in Prov. stuartii. One type of AAC(6) is chromosomally
encoded by Ser. marcescens, though not usually expressed.
The prevalence of resistance to amikacin remains low
(<5%) in many countries but can change rapidly with
increased usage of the drug. However, the spread of extended
spectrum β-lactamases belonging to the TEM and SHV families
may result in an increase in amikacin resistance that is
not associated with use, since most strains that produce such
enzymes also produce AAC(6′).
General Description
Amikacin was synthesized by Kawaguchi et al. of the Bristol-Banyu Research Institute in 1970 starting with kanamycin and the acyl moiety of butirosin. Its design is based on knowledge of the mechanisms of bacterial resistance to kanamycin and related compounds in which the 3 -hydroxyl group of the antibiotic is phosphorylated enzymatically. The acyl moiety in butirosin prevents this enzymatic inactivation.
Pharmacokinetics
Cmax 7.5 mg/kg intramuscular: c. 30 mg/L after 1 h
500 mg 30-min infusion: 35–50 mg/L end infusion
15 mg/kg 30-min infusion: >50 mg/L after 1 h
Plasma half-life: 2.2 h
Volume of distribution: 0.25–0.3 L/kg
Plasma protein binding: 3–11%
It is readily absorbed after intramuscular administration.
Rapid intravenous injection of 7.5 mg/kg produced concentrations
in excess of 60 mg/L shortly after injection.
Most pharmacokinetic parameters follow an almost linear
correlation when the once-daily doses (15 mg/kg) are compared
with the traditional 7.5 mg/kg twice daily. In patients on CAPD,
there was no difference in mean peak plasma concentration or
volume of distribution whether the drug was given intravenously or intraperitoneally. However, in patients with significant burn
injuries, doses should be increased to 20 mg/kg.
In infants receiving 7.5 mg/kg by intravenous injection,
peak plasma concentrations were 17–20 mg/L. No accumulation
occurred on 12 mg/kg per day for 5–7 days. There was
little change in the plasma concentration or the half-life (1.7
and 1.9 h) on the third and seventh days of a period over
which 150 mg/m2 was infused over 30 min every 6 h. When
the dose was raised to 200 mg/m2 the concentration never fell
below 8 mg/L. The plasma half-life was longer in babies of
lower birth weight and was still 5–5.5 h in babies aged 1 week
or older. The importance of dosage control in the neonate is
emphasized by the findings that there is an inverse relationship
between post-conception age and plasma elimination
half-life, though in extremely premature babies the weight of
the child is also a significant predictor of half-life.
Clinical Use
Severe infection (including septicemia, neonatal sepsis, osteomyelitis,
septic arthritis, respiratory tract, urinary tract, intra-abdominal, peritoneal
and soft tissue infections) caused by susceptible micro-organisms
Sepsis of unknown origin (combined with a β-lactam or anti-anaerobe
agent as appropriate).
Mycobacterial infection
Amikacin is principally used for the treatment of infections
caused by organisms resistant to other aminoglycosides
because of their ability to degrade them. Peak concentrations
on 15 mg/kg once daily administration should exceed
45 mg/L, and trough concentration of <5 mg/L should be
maintained to achieve therapeutic effects.
Safety Profile
Poison by intravenous,intraperitoneal, and intramuscular routes. Moderately toxicby intraperitoneal route. An experimental teratogen. Whenheated to decomposition it emits toxic fumes of NOx.
AMIKACIN Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 461 | 58 | Inquiry |
| Varanous Labs Pvt Ltd | +91-7036248882 +91-7036248882 | Hyderabad, India | 1541 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| M S Pharmaceuticals | 08048371760Ext 161 | Delhi, India | 3 | 58 | Inquiry |
| Rhyme Organics And Chemicals Limited | 08048372046Ext 929 | Hyderabad, India | 102 | 58 | Inquiry |
| T And D Trading Private Limited | 08069013895Ext 856 | Mumbai, India | 44 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
Related articles
- What is Amikacin?
- Amikacin is a semisynthetic aminoglycoside antibiotic. It was derived by the addition of the (S)-4-amino-2-hydroxybutyryl (AHB....
- Mar 11,2022
37517-28-5(AMIKACIN)Related Search:
1of4
chevron_right




