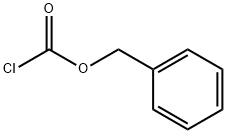
Benzyl chloroformate
- CAS No.
- 501-53-1
- Chemical Name:
- Benzyl chloroformate
- Synonyms
- CBZ;Benzyl carbonochloridate;BCF;Z-CL;BENZYLOXYCARBONYL CHLORIDE;Benzyl Chlorofomate;CBZ CHLORIDE;CARBOBENZOXY CHLORIDE;Z-CHLORIDE;CBZ-Cl (Z-Cl)
- CBNumber:
- CB7853678
- Molecular Formula:
- C8H7ClO2
- Molecular Weight:
- 170.59
- MOL File:
- 501-53-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/8/15 13:09:01
| Melting point | -20 °C |
|---|---|
| Boiling point | 103 °C20 mm Hg(lit.) |
| Density | 1.212 g/mL at 20 °C |
| vapor density | 1 (vs air) |
| vapor pressure | 1.39 psi ( 20 °C) |
| refractive index |
n |
| Flash point | 197 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | Miscible with ether, acetone and benzene. |
| form | Solution |
| color | slightly yellow to slightly pink |
| Odor | Irritating; sharp, penetrating. |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,1801 |
| Exposure limits |
ACGIH: TWA 1 ppm OSHA: TWA 1 ppm(5 mg/m3) NIOSH: IDLH 10 ppm; Ceiling 1 ppm(5 mg/m3) |
| Stability | Stable. Incompatible with water, oxidizing agents. Combustible. |
| CAS DataBase Reference | 501-53-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzyl chloroformate(501-53-1) |
| EPA Substance Registry System | Carbonochloridic acid, phenylmethyl ester (501-53-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS05,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H314-H335-H350-H410 | |||||||||
| Precautionary statements | P201-P261-P273-P280-P305+P351+P338-P310 | |||||||||
| Hazard Codes | T,C,N,F | |||||||||
| Risk Statements | 45-20-34-48/22-50/53-67-65-63-48/20-11-43-37-23 | |||||||||
| Safety Statements | 53-26-36/37/39-45-60-61-62-46-36/37 | |||||||||
| RIDADR | UN 2924 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LQ5860000 | |||||||||
| F | 19 | |||||||||
| Autoignition Temperature | 590 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 90 70 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | LD50 orally in Rabbit: 3000 mg/kg | |||||||||
| NFPA 704 |
|
Benzyl chloroformate price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.14688 | Benzyl chloroformate (stabilised) for synthesis | 501-53-1 | 250ML | ₹32610 | 2022-06-14 | Buy |
| TCI Chemicals (India) | B3021 | Benzyl Chloroformate min. 94.0 % | 501-53-1 | 25G | ₹2000 | 2022-05-26 | Buy |
| TCI Chemicals (India) | B3021 | Benzyl Chloroformate min. 94.0 % | 501-53-1 | 250G | ₹5000 | 2022-05-26 | Buy |
Benzyl chloroformate Chemical Properties,Uses,Production
Description
Benzyl chloroformate, also known as benzyl chlorocarbonate or Z-chloride, is the benzyl ester of chloroformic acid. It can be also described as the chloride of the benzyloxycarbonyl (Cbz or Z) group. In its pure form it is a water-sensitive oily colorless liquid, although impure samples usually appear yellow. It possesses a characteristic pungent odor and degrades in contact with water.
The compound was first prepared by Leonidas Zervas in the early 1930s who used it for the introduction of the benzyloxycarbonyl protecting group, which became the basis of the Bergmann-Zervas carboxybenzyl method of peptide synthesis he developed with Max Bergmann. This was the first successful method of controlled peptide chemical synthesis and for twenty years it was the dominant procedure used worldwide until the 1950s. To this day, benzyl chloroformate is often used for amine group protection.
Chemical Properties
colorless or light yellow oily liquid with rancid odor. soluble in ether, acetone, benzene and other organic solvents. It is used to protect amino groups in peptide synthesis.
Uses
Benzyl chloroformate is widely used as a reactive chemical intermediate in plastic, pharmaceutical, agricultural and organic chemicals. It is useful for the introduction of carboxybenzyl (cbz) protecting group for amines such as aniline in organic synthesis. It is also involved in the synthesis of 1,2,4-oxadiazoles.
Preparation
Benzyl chloroformate is prepared in the lab by treating benzyl alcohol with phosgene:
PhCH2OH + COCl2→ PhCH2OC(O)Cl + HCl
Phosgene is used in excess to minimise the production of the carbonate (PhCH2O)2C=O.
The use of phosgene gas in the lab preparation carries a very large health hazard, and has been implicated in the chronic pulmonary disease of pioneers in the usage of the compound such as Zervas.
Application
Benzyl chloroformate is used as a reagent in peptide synthesis to protect the amine functionality as the benzyloxycarbonyl (Cbz or Z) derivative.
Cbz-protected anilines were prepared directly from aromatic carboxylic acids, sodium azide and Cbz-Cl.
Protecting reagent in peptide synthesis.
General Description
Benzyl chloroformate appears as a colorless liquid with an acrid odor. Vapors irritate eyes and mucous membranes. Corrosive to metals and tissue. Long-term inhalation of low concentrations or short-term inhalation of high concentrations can result in adverse health effects.
Air & Water Reactions
Decomposes in moist air. Decomposes slowly in water to give corrosive hydrochloric acid and organic acids.
Reactivity Profile
Benzyl chloroformate decomposes slowly in water forming benzyl alcohol, HCl, and CO2. Gives off HCl fumes in moist air. Reacts with bases, both organic and inorganic. Attacks many metals especially in humid atmosphere [Handling Chemicals Safely 1980. p. 476]. Catalytic impurity incidents involving the iron catalyzed decomposition of benzoyl chloroformate have caused several explosions. The iron presumably comes from corrosion of steel storage tanks [Loss Prev. Bull., 1975, (003), 2]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Highly toxic, emits very toxic phosgene fumes at 100C. Irritant to eyes.
Health Hazard
Inhalation causes mucous membrane irritation. Eyes are irritated by excessive exposure to vapor. Liquid causes severe irritation of eyes and irritates skin. Ingestion causes irritation of mouth and stomach.
Safety Profile
Poison by ingestion andinhalation routes. A powerful corrosive irritant. Thermallyunstable. Will react with water or steam to produce toxic and corrosive fumes and heat. Iron salts catalyze theexplosive decomposition of the ester. When heated todecomp.
Purification Methods
The commercial material is usually better than 95% pure and may contain some toluene, benzyl alcohol, benzyl chloride and HCl. After long storage (e.g. two years at 4o, Greenstein and Winitz [The Chemistry of the Amino Acids Vol 2 p. 890, J Wiley and Sons NY, 1961] recommended that the liquid should be flushed with a stream of dry air, filtered and stored over sodium sulfate to remove CO2 and HCl which are formed by decomposition. It may further be distilled from an oil bath at a temperature below 85o because Thiel and Dent [Annalen 301 257 1898] stated that benzyloxycarbonyl chloride decarboxylates to benzyl chloride slowly at 100o and vigorously at 155o. Redistillation at higher vacuum below 85o yields material which shows no other peaks than those of benzyloxycarbonyl chloride by NMR spectroscopy. [Beilstein 6 IV 2278.] LACHRYMATORY and TOXIC.
Benzyl chloroformate Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| BASF India Limited | +91-2262785600 +91-2262785600 | Maharashtra, India | 209 | 58 | Inquiry |
| Honour Lab Limited | +919845977466 | Telangana, India | 164 | 58 | Inquiry |
| Paushak Limited | +91-2653007932 +91-9727736899 | Vadodara, India | 59 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| BASF India Limited | 91-22-67127600 | Maharashtra, India | 138 | 58 | Inquiry |
| Hermes Chemical Company Private Limited | +91 40 27715685 | Hyderabad, India | 15 | 58 | Inquiry |
| VASIN BIO LIFE SCIENCES PVT LTD | 9491644688 | Hyderabad, India | 2096 | 58 | Inquiry |
| Bridge Chem Pvt., Ltd. | 91-22-66611488 | Mumbai, India | 128 | 58 | Inquiry |
| Shivam Pharma Chemicals | 91-22-26403900 | Maharashtra, India | 656 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| JSK Chemicals | 58 |
| BASF India Limited | 58 |
| Honour Lab Limited | 58 |
| Paushak Limited | 58 |
| S D Fine Chem Limited | 58 |
| BASF India Limited | 58 |
| Hermes Chemical Company Private Limited | 58 |
| VASIN BIO LIFE SCIENCES PVT LTD | 58 |
| Bridge Chem Pvt., Ltd. | 58 |
| Shivam Pharma Chemicals | 58 |
501-53-1(Benzyl chloroformate)Related Search:
1of4
chevron_right




