NAGLY
- CAS No.
- 179113-91-8
- Chemical Name:
- NAGLY
- Synonyms
- NAGLY;NAGly, CID 5283389;Arachidonyl Glycine;ARACHIDONOYL GLYCINE;N-ARACHIDONYL GLYCINE;N-ARACHIDONOYL GLYCINE;N Arachidonylglycine,NArachidonylglycine;N-Arachidonoyl glycine (solution in ethanol);N-(1-OXO-5Z,8Z,11Z,14Z-EICOSATETRAENYL)GLYCINE;Glycine, N-[(5Z,8Z,11Z,14Z)-1-oxo-5,8,11,14-eicosatetraen-1-yl]-
- CBNumber:
- CB0232490
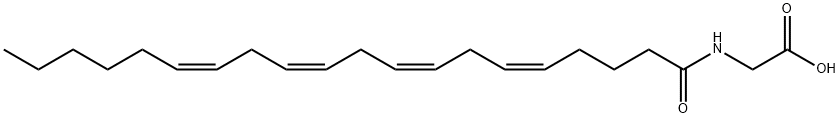
- Molecular Formula:
- C22H35NO3
- Molecular Weight:
- 361.52
- MOL File:
- 179113-91-8.mol
- Modify Date:
- 2023/6/30 15:45:59
| Boiling point | 560.9±50.0 °C(Predicted) |
|---|---|
| Density | 0.985±0.06 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | DMSO: >5 mg/mL, soluble |
| form | waxy solid |
| pka | 3.58±0.10(Predicted) |
| color | off-white |
| Sensitive | Air Sensitive |
| Stability | Stable for 2 years from date of purchase as supplied. Product is subject to oxidation. Solutions in degassed DMSO may be stored at -20°C for up to 1 month. |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H336-H351 | |||||||||
| Precautionary statements | P201-P302+P352-P305+P351+P338-P308+P313 | |||||||||
| Safety Statements | 22-24/25 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
NAGLY Chemical Properties,Uses,Production
Description
Arachidonoyl glycine (N-
Uses
Arachidonoyl glycine (N-arachidonyl glycine; NAGly) has been isolated from cell cultures treated with arachidonoyl ethanolamide (AEA; anandamide), from extracts of mammalian brain, and has also been synthesized as an analog of AEA for structure/activity testing. NAGly may be produced endogenously via oxidation of AEA, or by transacylation of arachidonoyl CoA. NAGly is reported to have analgesic activities in whole animal experiments. Since it seems to be a very poor ligand for the CB1 receptor, these effects are probably mediated via other signaling pathways.[Cayman Chemical]
Biological Activity
Endogenous anandamide-like compound. Lacks affinity for CB 1 receptors (K i > 10 μ M), VR1 receptors (EC 50 > 10 μ M) and anandamide transporters (IC 50 > 50 μ M) but causes hot-plate analgesia in mice when given orally, and suppresses tonic inflammatory pain. Also endogenous GlyT2 inhibitor.
NAGLY Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Alfa Chemistry | United States | 24072 | 58 | Inquiry | |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Shanghai Aladdin Biochemical Technology Co.,Ltd. | +86-18521732826 | China | 48467 | 58 | Inquiry |
| RD International Technology Co., Limited | 18024082417 | China | 9282 | 58 | Inquiry |
| SHANGHAI ZZBIO CO., LTD. | 18019219489 19921389125 | China | 12064 | 58 | Inquiry |
| Nantong QuanYi Biotechnology Co., Ltd | 0513-66337626 18051384581 | China | 4552 | 58 | Inquiry |
| Cayman Chemical Company | (800) 364-9897 | United States | 6618 | 81 | Inquiry |
| Shanghai EFE Biological Technology Co., Ltd. | 021-65675885 18964387627 | China | 9707 | 58 | Inquiry |
| Shenzhen Regent Biochemical Technology Co., Ltd. | 0755-0755-85201366 18938635012 | China | 9304 | 58 | Inquiry |





