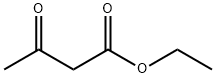
Ethyl acetoacetate
- CAS No.
- 141-97-9
- Chemical Name:
- Ethyl acetoacetate
- Synonyms
- ETHYL 3-OXOBUTANOATE;ACE;EAA;Ethyl acetoacetate, 99%, pure;ACETOACETIC ESTER;Ethyl acetyl acetate;Acetoacetic acid ethyl;Ethyl acetoacetate(EAA);Ethyl acetoacetate, extra pure;3-OXOBUTANOIC ACID ETHYL ESTER
- CBNumber:
- CB0301721
- Molecular Formula:
- C6H10O3
- Molecular Weight:
- 130.14
- MOL File:
- 141-97-9.mol
- Modify Date:
- 2024/7/9 21:58:15
| Melting point | −43 °C(lit.) |
|---|---|
| Boiling point | 181 °C(lit.) |
| Density | 1.029 g/mL at 20 °C(lit.) |
| vapor density | 4.48 (vs air) |
| vapor pressure | 1 mm Hg ( 28.5 °C) |
| FEMA | 2415 | ETHYL ACETOACETATE |
| refractive index |
n |
| Flash point | 185 °F |
| storage temp. | Store below +30°C. |
| solubility | 116 g/L (20°C) |
| pka | 11(at 25℃) |
| form | Liquid |
| color | APHA: ≤15 |
| Specific Gravity | 1.027~1.035 (20/4℃) |
| Relative polarity | 0.577 |
| PH | 4.0 (110g/l, H2O, 20℃) |
| Odor | Agreeable, fruity. |
| Odor Type | fruity |
| explosive limit | 1.0-54%(V) |
| Water Solubility | 116 g/L (20 ºC) |
| JECFA Number | 595 |
| Merck | 14,3758 |
| BRN | 385838 |
| Dielectric constant | 15.0(Ambient) |
| Stability | Stable. Incompatible with acids, bases, oxidizing agents, reducing agents, alkali metals. Combustible. |
| InChIKey | XYIBRDXRRQCHLP-UHFFFAOYSA-N |
| LogP | 0.8 at 20℃ |
| CAS DataBase Reference | 141-97-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, 3-oxo-, ethyl ester(141-97-9) |
| EPA Substance Registry System | Ethyl acetoacetate (141-97-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H227-H319-H303 | |||||||||
| Precautionary statements | P210e-P280a-P305+P351+P338-P337+P313-P501a-P210-P264-P280-P305+P351+P338+P337+P313-P370+P378-P403+P235-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36 | |||||||||
| Safety Statements | 26-24/25 | |||||||||
| RIDADR | UN 1993 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AK5250000 | |||||||||
| Autoignition Temperature | 580 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3.2 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29183000 | |||||||||
| Toxicity | LD50 orally in rats: 3.98 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Ethyl acetoacetate price More Price(27)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W241512 | Ethyl acetoacetate natural, ≥97%, FG | 141-97-9 | 1SAMPLE-K | ₹5347.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W241512 | Ethyl acetoacetate natural, ≥97%, FG | 141-97-9 | 100G | ₹7241.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W241512 | Ethyl acetoacetate natural, ≥97%, FG | 141-97-9 | 1KG | ₹21736.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W241512 | Ethyl acetoacetate natural, ≥97%, FG | 141-97-9 | 5KG | ₹92304.78 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W241504 | Ethyl acetoacetate ≥99%, FCC, FG | 141-97-9 | 1SAMPLE-K | ₹5141.88 | 2022-06-14 | Buy |
Ethyl acetoacetate Chemical Properties,Uses,Production
Description
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and amino pyrine, and vitamin B1; as well as the manufacture of dyes, inks, lacquers, perfumes, plastics, and yellow paint pigments. Alone, it is used as a flavoring for food.
Chemical Properties
Ethyl 3-Oxobutanoate is a colorless liquid with a fruity, ethereal, sweet odor reminiscent of green apples. It is used to create fresh, fruity top notes in feminine fine fragrances. Ethyl acetoacetate occurs in flavors of natural materials such as coffee, strawberries, and yellow passion fruits.
Occurrence
Naturally occurring in strawberry, coffee, sherry, passion fruit juice (yellow), babaco fruit (Carica pentagona Heilborn) and bread.
Uses
Ethyl acetoacetate (EAA) is used as starting material for the syntheses of alpha-substituted acetoacetic esters and cyclic compounds, e.g. pyrazole, pyrimidine and coumarin derivatives as well as intermediate for vitamins and pharmaceuticals. Product Data Sheet
Production Methods
Ethyl acetoacetate is manufactured through a reaction of high-purity ethyl acetate with sodium, followed by neutralization with sulfuric acid.
Preparation
Ethyl acetoacetate is produced industrially by treatment of diketene with ethanol.
The preparation of ethyl acetoacetate is a classic laboratory procedure . It is prepared via the Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol.
Definition
This compound is a tautomer at room temperature consisting of about 93% keto form and 7% enol form.
General Description
A colorless liquid with a fruity odor. Flash point 185°F. Boiling point 365°F. May cause adverse health effects if ingested or inhaled. May irritate to skin, eyes and mucous membranes. Used in organic synthesis and in lacquers and paints.
Air & Water Reactions
Flammable.
Reactivity Profile
Ethyl acetoacetate, a beta-keto ester, is more reactive than many esters. Undergoes an exothermic cleavage reaction in the presence of concentrated base. Reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Mixing with 2,2,2-tris(bromomethyl)ethanol and zinc led to an explosion [US Patent 3 578 619, Crotonaldehyde may rapidly polymerize with Ethyl acetoacetate (Soriano, D.S. et al. 1988. Journal of Chemical Education 65:637.).1971].
Hazard
Toxic by ingestion and inhalation; irritant to skin and eyes.
Health Hazard
Liquid may cause mild irritation of eyes.
Chemical Reactivity
Ethyl acetoacetate is subject to Keto - enol tautomerism. Ethyl acetoacetate is often used in the acetoacetic ester synthesis similar to diethyl malonate in the malonic ester synthesis or the Knoevenagel condensation. The protons alpha to carbonyl groups are acidic, and the resulting carbanion can undergo nucleophilic substitution. A subsequent thermal decarboxylation is also possible.Similar to the behavior of acetylacetone, the enolate of ethyl acetoacetate can also serve as a bidentate ligand. For example, it forms purple coordination complexes with iron (III) salts :
Ethyl acetoacetate can also be reduced to ethyl 3-hydroxy butyrate.
Safety Profile
eye irritant. Combustible liquid when exposed to heat or flame; can react with oxidzing materials. Explosive reaction when heated with Zn + tribromoneopentyl alcohol or 2,2,2 tris(bromomethy1)ethanol. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Purification Methods
Shake the ester with small amounts of saturated aqueous NaHCO3 (until no further effervescence), then with water. Dry it with MgSO4 or CaCl2 and distil it under reduced pressure. [Beilstein 3 IV 1528.]
Ethyl acetoacetate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Vardhaman P Golechha | 9704422000 | Telangana, India | 294 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 83 | 58 | Inquiry |
| AMINO ORGANICS | +919866989891 | Hyderabad, India | 275 | 58 | Inquiry |
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Sri Neelima Laboratories | +91-7981733602 +91-9849736989 | Telangana, India | 205 | 58 | Inquiry |
| Laxmi Organic Industries Ltd | +91-2249104444 +91-2249104444 | Maharashtra, India | 23 | 58 | Inquiry |
| Jay Chem Marketing | 08048964412 | Mumbai, India | 231 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| ADVENT CHEMBIO PRIVATE LIMITED | 58 |
| Vardhaman P Golechha | 58 |
| Soham Chemical Industries | 58 |
| AMINO ORGANICS | 58 |
| REDDY N REDDY PHARMACEUTICALS | 58 |
| JSK Chemicals | 58 |
| Sri Neelima Laboratories | 58 |
| Laxmi Organic Industries Ltd | 58 |
| Jay Chem Marketing | 58 |
Related articles
- Ethyl Acetoacetate: A Versatile Compound for Biofilm Inhibition and Organic Synthesis
- Ethyl acetoacetate inhibits bacterial biofilm and is vital in organic synthesis, aiding pharmaceutical preparation due to taut....
- May 16,2024
141-97-9(Ethyl acetoacetate)Related Search:
1of4
chevron_right




