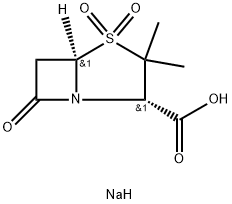
Sulbactam sodium
- CAS No.
- 69388-84-7
- Chemical Name:
- Sulbactam sodium
- Synonyms
- Sodium Sodium;Sulbatam Sodium;SODIUM SULBACTAM;Sterile Sulbactam sodium;(2S,5R);CP-45899-2;Shu ba sodium;sulbacyam sodium;SULBACTAM SODIUM;cp45899sodiumsalt
- CBNumber:
- CB0370356
- Molecular Formula:
- C8H12NNaO5S
- Molecular Weight:
- 257.24
- MOL File:
- 69388-84-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/12/3 16:17:04
| Melting point | >230°C (dec.) |
|---|---|
| storage temp. | -20°C Freezer, Under Inert Atmosphere |
| solubility | Freely soluble in water, sparingly soluble in ethyl acetate, very slightly soluble in ethanol (96 per cent). It is freely soluble in dilute acids. |
| form | Solid |
| color | White |
| InChIKey | NKZMPZCWBSWAOX-IBTYICNHSA-M |
| CAS DataBase Reference | 69388-84-7(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-24-36/37-45-24/25 |
| HS Code | 29349990 |
| Toxicity | LD50 ivn-rat: 6500 mg/kg NKRZAZ 32(Suppl 4),97,84 |
Sulbactam sodium Chemical Properties,Uses,Production
Description
Sulbactam sodium is a parenterally-active, β-lactamase inhibitor recently introduced as a 1: 1 combination product with cefoperazone. Like clavulanic acid, the first agent of this type to b e introduced, sulbactam enhances the effectiveness of β-lactam antibiotics against resistant strains.
Chemical Properties
White Solid
Uses
A semi-synthetic β-lactamase inhibitor. It is used in combination with β-lactam antibiotics as antibacterial.
General Description
Sulbactam was synthesized by Pfizer Research Laboratories in 1977 in the course of screening for β-lactamase inhibitors. It shows strong activity against penicillinase and moderate activity against cephalosporinase. Sulbactam itself shows activity against some gramnegative bacteria but no activity against most pathogenic bacteria. The use of sulbactam in combination with cefoperazone, which is partially hydrolyzed by penicillinase, is under study along with its use as an esterified complex with ampicillin (sultamicillin) for therapy of cefoperazone-ampicillin-resistant infections.
Safety Profile
Poison by intravenous route. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and SOx
Sulbactam sodium Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6202 | 58 | Inquiry |
| AUROBINDO PHARMA LTD | +91 (40) 6672 1200 | New Delhi, India | 209 | 58 | Inquiry |
| AUROBINDO PHARMA LIMITED | +91 (40) 6672 1200 | New Delhi, India | 144 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Uni Trade Agencies | 08068442234Ext 578 | Mumbai, India | 10 | 58 | Inquiry |
| CLEANCHEM LABORATORIES LLP | +91 98921 69560 | New Delhi, India | 325 | 58 | Inquiry |
| APITORIA PHARMA PRIVATE LTD | +91 40 6672 5000 | Chongqing, India | 110 | 58 | Inquiry |
69388-84-7(Sulbactam sodium)Related Search:
1of4
chevron_right




