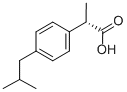
(S)-(+)-Ibuprofen
- CAS No.
- 51146-56-6
- Chemical Name:
- (S)-(+)-Ibuprofen
- Synonyms
- DEXIBUPROFEN;(S)-IBUPROFEN;(S)-2-(4-Isobutylphenyl)propanoic acid;Seractil;d-Ibuprofen;Ramelteon-7;exibuprofen;Deibuprofen;(+)-Ibuprofen;Dextrobuprofen
- CBNumber:
- CB0699655
- Molecular Formula:
- C13H18O2
- Molecular Weight:
- 206.28
- MOL File:
- 51146-56-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/13 17:03:25
| Melting point | 49-53 °C(lit.) |
|---|---|
| alpha | 57 º (c=2, EtOH) |
| Boiling point | 285.14°C (rough estimate) |
| Density | 1.0364 (rough estimate) |
| refractive index | 59 ° (C=2, EtOH) |
| Flash point | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 1.5 mg/mL |
| pka | 4.41±0.10(Predicted) |
| form | solid |
| color | white |
| optical activity | [α]20/D +59°, c = 2 in ethanol |
| Water Solubility | insoluble |
| BRN | 3590022 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | HEFNNWSXXWATRW-JTQLQIEISA-N |
| CAS DataBase Reference | 51146-56-6(CAS DataBase Reference) |
(S)-(+)-Ibuprofen price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 375160 | (S)-(+)-Ibuprofen ReagentPlus?, 99% | 51146-56-6 | 1G | ₹3788.75 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 375160 | (S)-(+)-Ibuprofen ReagentPlus?, 99% | 51146-56-6 | 5G | ₹11214.7 | 2022-06-14 | Buy |
| TCI Chemicals (India) | I0549 | (S)-(+)-Ibuprofen min. 98.0 % | 51146-56-6 | 1G | ₹3700 | 2022-05-26 | Buy |
| TCI Chemicals (India) | I0549 | (S)-(+)-Ibuprofen min. 98.0 % | 51146-56-6 | 5G | ₹10100 | 2022-05-26 | Buy |
(S)-(+)-Ibuprofen Chemical Properties,Uses,Production
Description
Dexibuprofen, the S-(+)-isomer of the widely used NSAID agent ibuprofen, was launched in Austria for the treatment of rheumatoid arthritis. While the racemic compound is commonly used clinically, the antiinflammatory activity is mediated via the S-isomer by inhibition of prostaglandin synthesis. It has also been demonstrated that the R-isomer is converted to the Santipode in vivo via a CoA thioester intermediate. Since CoA plays a pivotal role in intermediary metabolism and maintenance of the [acyl-CoA], generation of R-ibuprofen-CoA competitively inhibits many CoA-dependent reactions, which consequently produces perturbations of hepatocyte Intermediary metabolism and mitocondrial function. Pure S-ibuprofen usage, therefore, is preferred allowing a reduction in dosage level and an improved side effect profile.
Chemical Properties
Colourless, Crystalline Solid
Uses
A nonsteroidal anti-inflammatory drug (NSAID); activity resides primarily in the (S)-isomer
General Description
(S)-(+)-Ibuprofen is the enantiomer associated with the anti-inflammatory action of ibuprofen, which is widely used as a nonsteroidal anti-inflammatory drug in racemic form.
Biological Activity
Non-steroidal anti-inflammatory drug (NSAID) that inhibits cyclooxygenase 1 and cyclooxygenase 2 (IC 50 values are 12 and 80 μ M respectively). Active isomer of ibuprofen.
Purification Methods
Crystallise the (+) and (-) acids from EtOH or aqueous EtOH. The racemate which crystallises from pet ether with m 75-77o is sparingly soluble in H2O and has IR (film) 1705 (C=O), 2300—3700 (OH broad)cm-1. It is used as a non-steroidal anti-inflammatory. [Shiori et al. J Org Chem 43 2936 1978, Kaiser et al. J Pharm Sci 65 269 1976, J Pharm Sci 81 221 1992, Freer Acta Cryst (C) 49 1378 1993 for the (S+)-enantiomer.]
(S)-(+)-Ibuprofen Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AVD pharmaceuticals Pvt Ltd | +919860835260 | Pune, India | 102 | 58 | Inquiry |
| SMS Pharmaceuticals Ltd | +91-8179850881 +91-9100092121 | Telangana, India | 22 | 58 | Inquiry |
| Arch Pharmalabs Ltd | +91-2242871210 +91-2242871210 | Maharashtra, India | 51 | 58 | Inquiry |
| Adani Wilmar Limited | +91-7383819375 +91-9099656790 | Gujarat, India | 34 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| IOL Chemicals And Pharmaceuticals Limited | +91-9878429978 +91-9878996045 | Punjab, India | 30 | 58 | Inquiry |
| Noven Lifesciences Pvt Limited | +91-9030209898 +91-9848878833 | Telangana, India | 21 | 58 | Inquiry |
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| Festiva Pharma | 08048372775Ext 728 | Gujarat, India | 238 | 58 | Inquiry |
| Kavya Pharma | 08048981075 | Gujarat, India | 55 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| AVD pharmaceuticals Pvt Ltd | 58 |
| SMS Pharmaceuticals Ltd | 58 |
| Arch Pharmalabs Ltd | 58 |
| Adani Wilmar Limited | 58 |
| Ralington Pharma | 58 |
| IOL Chemicals And Pharmaceuticals Limited | 58 |
| Noven Lifesciences Pvt Limited | 58 |
| AKASH PHARMA EXPORTS | 58 |
| Festiva Pharma | 58 |
| Kavya Pharma | 58 |
51146-56-6((S)-(+)-Ibuprofen )Related Search:
1of4
chevron_right





