Nitrocefin
- CAS No.
- 41906-86-9
- Chemical Name:
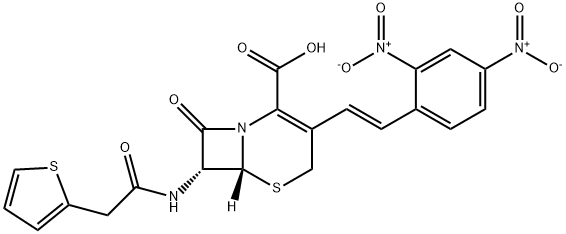
- Nitrocefin
- Synonyms
- 3-[(E)-2-(2,4-Dinitrophenyl)vinyl]-8-oxo-7-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;NITROCEFIN;Nitrocefin (>Nitrocefin (>90%);Cefnitrothiophene;3-(2,4-DINITROSTYRYL)-(6R, 7R)-7-(2-THIENYLACETAMIDO)-CEPH-3-EM-4 CARBOXYLIC ACID, E-ISOMER;(6R,7R)-7-[[(E)-4-(2,4-Dinitrophenyl)-2-(2-thienyl)but-3-enoyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;(6R)-3-[(E)-2-(2,4-Dinitrophenyl)ethenyl]-8-oxo-7α-[(2-thienylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(1E)-2-(2,4-dinitrophenyl)ethenyl]-8-oxo-7-[(2-thienylacetyl)amino]-, (6R,7R)-;(6R,7R)-3-[(E)-2-(2,4-dinitrophenyl)ethenyl]-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- CBNumber:
- CB1322600
- Molecular Formula:
- C21H16N4O8S2
- Molecular Weight:
- 516.5
- MOL File:
- 41906-86-9.mol
- Modify Date:
- 2024/7/2 8:55:00
| Melting point | 103-113° (dec); mp 167-169° (dec) (Lee) |
|---|---|
| alpha | D20 -224° (c = 1.0 in dioxane) |
| Boiling point | 872.0±65.0 °C(Predicted) |
| Density | 1.67±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; DMSO:PBS (pH 7.2) (1:20): 0.04 mg/ml |
| form | A crystalline solid |
| pka | 2.50±0.50(Predicted) |
| color | Yellow to orange |
| Stability | Hygroscopic, Unstable in Solution |
Nitrocefin Chemical Properties,Uses,Production
Description
The generation of β-
Chemical Properties
Nitrocefin is the chromogenic cephalosporin that acts as an excellent β-lactamase substrate. It exhibits a rapid distinctive color change from yellow (max at pH 7.0 = 390 nm) to red (max at pH 7.0 = 486 nm) as the amide bond in the beta-lactam ring is hydrolyzed by a β-lactamase. It is sensitive to hydrolysis by all known lactamases produced by Gram-positive and Gram-negative bacteria.
Nitrocefin (Yellow) --β-lactamase-->Product (Red) (OD486nm)
Solution preparation and color change before and after β-lactamase exposure
(A) Concentrated nitrocefin (10.0 mg/mL) in DMSO before dilution with PBS buffer. (B) Nitrocefin diluted with PBS buffer
to working concentration (1.0 mg/mL). The yellow color is indicative of intact, undegraded nitrocefin. (C) 25 units of betalactamase dropped on top of nitrocefin (1.0 mg/mL in PBS). The red color is the result of beta-lactamase mediated
cleavage of the nitrocefin. (D) Vortexed mixture of contents shown in picture (C).
Uses
Nitrocefin is a chromogenic β-lactamase substrate that undergoes colour change from yellow to red as the amide bond in the β-Lactam ring is hydrolyzed by β-lactamase. Nitrocefin undergoes colour chang es induced by lactamases produced by Gram-positive and Gram-negative bacteria. Several studies have utilized the colour changing properties of Nitrocefin for the detection of β-lactamase activity from bacterial cell extracts by isoelectric focusing and spectroscopy. Nitrocefin has also been used in studies involving β-lactamase resistant antibiotics.
Preparation
Nitrocefin is a key reagent for high and low throughput assays of the activities of penicillin-binding proteins (PBPs) and β-lactamases, the former used for discovery of antibiotics and the latter for inhibitors of resistance determinants for β-lactam antibiotics. This compound is commercially available but is prohibitively expensive because of the circuitous routes to its synthesis. We describe herein a three-step synthesis of nitrocefin that gives an overall yield of 44%. This is a practical route to the synthesis of this key reagent for drug discovery.
A Practical Synthesis of Nitrocefin
Biological Functions
In determination of b-lactamase activity in biological samples.
Nitrocefin is a colorless or faint yellow cephalosporin antimicrobial that is hydrolyzed rapidly by most beta-lactamases.The hydrolysis product is pink.The bacterium to be tested is applied to a paper disk containing nitrocefin. A pink color developing within minutes (positive test) indicates a beta-lactamaseproducing bacterium.
Nitrocefin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SRI ANAGHA BIOSCIENCES PVT LTD | +91-9959644262 +91-9959644262 | Telangana, India | 25 | 58 | Inquiry |
| Shanghai QiXin New Materials Technology Co.,Ltd | +8615116235351 | China | 16 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15395 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23556 | 58 | Inquiry |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | China | 2905 | 58 | Inquiry |
41906-86-9(Nitrocefin)Related Search:
1of2
chevron_right





