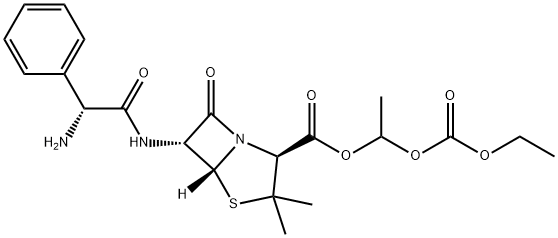
BACAMPICILLIN
- CAS No.
- 50972-17-3
- Chemical Name:
- BACAMPICILLIN
- Synonyms
- Carampicillin;BACAMPICILLIN;BACAMPICILLIN USP/EP/BP;BACAMPICILLIN HCL USP(CRM STANDARD);Bacampicillin (base and/or unspecified salts);6α-[[(R)-2-Amino-2-phenylacetyl]amino]penicillanic acid 1-[(ethoxycarbonyl)oxy]ethyl ester;(2S,5R,6R)-6-[[(2R)-2-Amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 1-(ethoxycarbonyloxy)ethyl ester;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2R)-aminophenylacetyl]amino]-3,3-dimethyl-7-oxo-, 1-[(ethoxycarbonyl)oxy]ethyl ester, (2S,5R,6R)-;(2S,5R,6R)-6-[[(2R)-2-amino-1-oxo-2-phenylethyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 1-ethoxycarbonyloxyethyl ester;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, 1-[(ethoxycarbonyl)oxy]ethyl ester, [2S-[2α,5α,6β(S*)]]-
- CBNumber:
- CB1343108
- Molecular Formula:
- C21H27N3O7S
- Molecular Weight:
- 465.52
- MOL File:
- 50972-17-3.mol
- Modify Date:
- 2023/5/21 10:59:17
| Boiling point | 678.4±55.0 °C(Predicted) |
|---|---|
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | pKa 6.8 (Uncertain) |
| CAS DataBase Reference | 50972-17-3 |
BACAMPICILLIN Chemical Properties,Uses,Production
Definition
ChEBI: A penicillanic acid ester that is the 1-ethoxycarbonyloxyethyl ester of ampicillin. It is a semi-synthetic, microbiologically inactive prodrug of ampicillin.
Metabolism
Although comparatively well absorbed (30–55%), the oral efficacy of ampicillin for systemic infections can be enhanced significantly through the preparation of pro-drugs. In contrast to ampicillin itself, which is amphoteric, bacampicillin is a weak base and is very well absorbed in the duodenum (80–98%). Enzymatic ester hydrolysis in the gut wall liberates carbon dioxide and ethanol, followed by spontaneous loss of acetaldehyde and production of ampicillin. The acetaldehyde is metabolized oxidatively by alcohol dehydrogenase to produce acetic acid, which joins the normal metabolic pool.
BACAMPICILLIN Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| OCEAN TRADING CORPORATION | +91(22) 24921669 | New Delhi, India | 6211 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| BOC Sciences | 16314854226; +16314854226 | United States | 19743 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131980 | 58 | Inquiry |
| American Custom Chemicals Corporation | 858 201 6118 | United States | 6826 | 51 | Inquiry |
| 3B Scientific Corporation | 847 281 9822 | United States | 6971 | 56 | Inquiry |
| A.T.CHEMICAL | 0755-86655561 81899190 | China | 6594 | 51 | Inquiry |
| Jiangsu Aikon Biopharmaceutical R&D co.,Ltd. | 025-66113011 18112977050 | China | 10529 | 58 | Inquiry |
50972-17-3(BACAMPICILLIN)Related Search:
1of3
chevron_right




