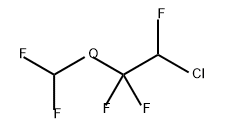
Enflurane
- CAS No.
- 13838-16-9
- Chemical Name:
- Enflurane
- Synonyms
- 347;C 347;Efrane;Alyrane;Ethrane;ohio347;Ohio 347;ENFLURAN;ENFLURANE;NSC-115944
- CBNumber:
- CB1361418
- Molecular Formula:
- C3H2ClF5O
- Molecular Weight:
- 184.49
- MOL File:
- 13838-16-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:00
| Boiling point | 56 °C |
|---|---|
| Density | 1.517 |
| refractive index | 1.303 |
| Flash point | 56-57°C |
| storage temp. | 2-8°C |
| solubility | Chloroform (Soluble), DMSO (Sparingly), Dichloromethane (Sparingly), Methanol (S |
| form | Liquid |
| color | Colorless to light yellow |
| Specific Gravity | 1.517 |
| Merck | 14,3581 |
| BRN | 1737129 |
| Exposure limits | TLV-TWA 570 mg/m3 (75 ppm) (ACGIH). |
| InChIKey | JPGQOUSTVILISH-UHFFFAOYSA-N |
| CAS DataBase Reference | 13838-16-9(CAS DataBase Reference) |
| EPA Substance Registry System | Enflurane (13838-16-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | F,T,Xi | |||||||||
| Risk Statements | 36 | |||||||||
| Safety Statements | 23-26-36-39 | |||||||||
| RIDADR | UN 3334 | |||||||||
| OEL | Ceiling: 2 ppm (15.1 mg/m3) [60-minute] [*Note: REL for exposure to waste anesthetic gas.] | |||||||||
| RTECS | KN6800000 | |||||||||
| Hazard Note | Flammable/Toxic | |||||||||
| HS Code | 2909191800 | |||||||||
| Toxicity | LD50 oral in rat: 5450uL/kg | |||||||||
| NFPA 704 |
|
Enflurane price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | CDS019920 | 2-Chloro-1,1,2-trifluoroethyl difluoromethyl ether AldrichCPR | 13838-16-9 | 1G | ₹6170.25 | 2022-06-14 | Buy |
| ALFA India | ALF-L16725-14 | 2-Chloro-1,1,2-trifluoroethyl difluoromethyl ether, 97% | 13838-16-9 | 25g | ₹37969 | 2022-05-26 | Buy |
| ALFA India | ALF-L16725-06 | 2-Chloro-1,1,2-trifluoroethyl difluoromethyl ether, 97% | 13838-16-9 | 5g | ₹10489 | 2022-05-26 | Buy |
Enflurane Chemical Properties,Uses,Production
Chemical Properties
Enflurane is a clear, colorless liquid that easily turns into a nonflammable gas. Mild, sweet odor
Uses
Clinical anesthetic.
Definition
ChEBI: An ether in which the oxygen atom is connected to 2-chloro-1,1,2-trifluoroethyl and difluoromethyl groups.
General Description
Enflurane is a volatile liquid (bp=56.5°C) with a blood:gas partition coefficient of 1.8 and an MAC of 1.68%.Approximately 2% to 8% of the drug is metabolized primarilyat the chlorofluoromethyl carbon. Little chlorofluoroaceticacid is produced suggesting minor metabolism at thedifluoromethyl carbon. Difluoromethoxydifluoroacetate andfluoride ion have been reported as metabolites. Enfluranemay increase heart rate, cause cardiac arrhythmias, increasecerebral blood flow, and increase intracranial pressure but allto a smaller degree than halothane. Enflurane also causeselectroencephalographic (EEG) patterns consistent withelectrical seizure activity. It has caused tonic–clonic convulsiveactivity in patients when used at high concentrations orduring profound hypocarbic periods. Enflurane is thereforenot recommended in patients with seizure disorders.
Reactivity Profile
The material 2-CHLORO-1,1,2-TRIFLUOROETHYL DIFLUOROMETHYL ETHER is incompatible with the following oxidizing materials, peroxides, combustible materials. Although nonflammable, a fire may cause 2-CHLORO-1,1,2-TRIFLUOROETHYL DIFLUOROMETHYL ETHER to decompose to toxic compounds including phosgene, hydrogen chloride, and hydrogen fluoride. Decomposes slowly in the light.
Hazard
Volatile with anesthetic properties, but nonflammable. Cardiac and central nervous system impairment. Questionable carcinogen.
Fire Hazard
Noncombustible liquid; flash point >94°C (200°F); low reactivity. Pressure buildup in a closed bottle may occur at elevated temper atures.
Clinical Use
Enflurane was introduced into medical practice in the United States in 1973 and is a clear, colorless, nonflammable general liquid with a mild, sweet odor. Although relatively stable chemically, enflurane does not attack aluminum, copper, iron, or brass and is soluble in rubber (partition coefficient = 74), which can prolong induction/recovery times, as seen with halothane.Enflurane has an intermediate solubility in blood and significant potency. Most of its pharmacological properties are similar to those of halothane, although there may be slightly less nausea, vomiting, arrhythmias, and postoperative shivering than observed with halothane. High concentrations of enflurane, however, are more likely to produce convulsions and circulatory depression. Enflurane also relaxes the uterus and, thus, should not be used as an anesthetic during labor. Metabolism via CYP2E1 accounts for 2% of an inhaled dose and includes transformation to the fluoride ion and fluoromethoxydifluoroacetic acid. During recovery, enflurane leaves the fatty tissues rapidly and, therefore, is not available for a prolonged period of time for significant metabolism to proceed.
Safety Profile
Mildly toxic by inhalation, ingestion, and subcutaneous routes. Human systemic effects by inhalation: decreased urine volume or anuria. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. An eye irritant. Questionable carcinogen with experimental carcinogenic data. An anesthetic. When heated to decomposition it emits very toxic fumes of Fand Cl-. See also ETHERS.
Potential Exposure
FDA-proprietary drug, used as an anesthetic (gas). Axphyxiant
Shipping
UN1851 Medicine, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. Cylinders must be transported in a secure upright position, in a wellventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Decomposes on heating, forming toxic and corrosive fumes of hydrogen chloride, hydrogen fluoride, and phosgene. Decomposes in strong sunlight
Waste Disposal
Return refillable compressed gas cylinders to supplier
Enflurane Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21667 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9338 | 55 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10311 | 58 | Inquiry |
| Shanghai Worldyang Chemical Co.,Ltd. | +86-021-56795779 +8613651600618 | China | 1080 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29825 | 58 | Inquiry |
Related articles
- Usage and Dosage of Enflurane
- Enflurane is used for compound general anesthesia. Enflurane is compatible with a variety of intravenous general anesthesia an....
- Oct 28,2019
13838-16-9(Enflurane)Related Search:
1of4
chevron_right




