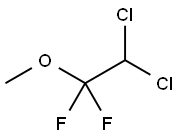
methoxyflurane
- CAS No.
- 76-38-0
- Chemical Name:
- methoxyflurane
- Synonyms
- MOZ;MOF;KAT8;hMOF;MYST1;KAT6A;MYST3;ZNF220;DA-759;RUNXBP2
- CBNumber:
- CB6314297
- Molecular Formula:
- C3H4Cl2F2O
- Molecular Weight:
- 164.97
- MOL File:
- 76-38-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/19 15:37:50
| Melting point | -36°C |
|---|---|
| Boiling point | 103 °C |
| Density | 1.4262 |
| refractive index | 1.386 |
| Flash point | 37°C |
| storage temp. | -70°C |
| solubility | Chloroform (Sparingly) |
| form | liquid |
| color | Clear |
| Water Solubility | Miscible with alcohol, acetone, chloroform, ether, fixed oils and benzene. Immiscible with water. |
| Merck | 14,5994 |
| BRN | 1737766 |
| Exposure limits | No exposure limit is set. Based on comparison with related compounds, a TLV-TWA of 675 mg/m3 (100 ppm) is recommended. |
| Stability | Stability |
| CAS DataBase Reference | 76-38-0(CAS DataBase Reference) |
| EPA Substance Registry System | Methoxyflurane (76-38-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H226-H319-H341 | |||||||||
| Precautionary statements | P210-P241-P308+P311 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 10 | |||||||||
| Safety Statements | 23-24/25 | |||||||||
| RIDADR | 3271 | |||||||||
| OEL | Ceiling: 2 ppm (13.5 mg/m3) [60-minute] [*Note: REL for exposure to waste anesthetic gas.] | |||||||||
| RTECS | KN7820000 | |||||||||
| Hazard Note | Irritant | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2909191800 | |||||||||
| Toxicity | One of the most potent of the inhalational anesthetics, having a very high blood-gas partition coefficient and low vapor pressure at room temperature. Methoxyflurane is metabolized to a great extent (about 50-70%) in the liver and, as a consequence, there may be release of high concentrations of fluoride, sufficient to exceed the threshold for renal damage. Its use for sustained anesthesia is limited because of this renal toxicity and was discontinued around 1980. | |||||||||
| NFPA 704 |
|
methoxyflurane price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SRP5220 | KAT8 (2-467), GST tagged human recombinant, expressed in baculovirus infected Sf9 cells, ≥70% (SDS-PAGE), buffered aqueous glycerol solution | 2226028-18-6 | 20μG | ₹33388.8 | 2022-06-14 | Buy |
| ALFA India | ALF-L17285-14 | 2,2-Dichloro-1,1-difluoroethyl methyl ether, 97% | 76-38-0 | 25g | ₹18348 | 2022-05-26 | Buy |
| ALFA India | ALF-L17285-06 | 2,2-Dichloro-1,1-difluoroethyl methyl ether, 97% | 76-38-0 | 5g | ₹4605 | 2022-05-26 | Buy |
methoxyflurane Chemical Properties,Uses,Production
Chemical Properties
colourless liquid
Uses
Methoxyflurane is a very potent and highly lipid soluble anesthetic agent. Methoxyflurane causes deep sedation and it has been used as a patient controlled analgesic for painful procedures in children. Methoxyflurane is a significant respiratory depressant.
Definition
ChEBI: An ether in which the two groups attached to the central oxygen atom are methyl and 2,2-dichloro-1,1-difluoroethyl.
Biological Functions
Methoxyflurane (Penthrane) is the most potent inhalational agent available, but its high solubility in tissues limits its use as an induction anesthetic. Its pharmacological properties are similar to those of halothane with some notable exceptions. For example, since methoxyflurane does not depress cardiovascular reflexes, its direct myocardial depressant effect is partially offset by reflex tachycardia, so arterial blood pressure is better maintained. Also, the oxidative metabolism of methoxyflurane results in the production of oxalic acid and fluoride concentrations that approach the threshold of causing renal tubular dysfunction. Concern for nephrotoxicity has greatly restricted the use of methoxyflurane.
General Description
Methoxyflurane is a volatile liquid (bp=105°C) with a highblood:gas partition coefficient and thus a slow induction andprolonged recovery. Approximately 75% of the drug undergoesmetabolism yielding dichloroacetate, difluoromethoxyacetate,oxalate, and fluoride ions. The intrarenal inorganicfluoride concentration, as a result of renal defluorination, maybe responsible for the nephrotoxicity seen with methoxyflurane.Both the concentration of F- generated and the durationfor which it remained elevated were factors in the developmentof methoxyflurane nephrotoxicity. Methoxyfluranewas removed from the U.S. market in 2000 because of saferalternatives. Both isoflurane and enflurane produce less fluorideion upon metabolism than methoxyflurane.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,2-DICHLORO-1,1-DIFLUOROETHYL METHYL ETHER may be sensitive to prolonged exposure to light.
Health Hazard
Methoxyflurane exhibited low to very lowacute toxicity via inhalation, slightly lowerthan that of ethrane. Oral toxicity was low tomoderate depending on the species. Inhala tion of its vapors at 1.5–2% by volumeconcentrations in air can cause anesthesia inhumans. The toxic symptoms are similar tothose of ethrane, and the target organs areprimarily the central nervous system, kidney,and liver. At subanesthetic concentrations of0.3–0.5% by volume in air, its exposure tohumans for 1 hour resulted in the onset oflow toxicity. The sites of biological effectswere in the kidney.
LC50 value, inhalation (mice): 17,500 ppm/2 hr
LD50 value, oral (mammals): 3600 mg/kg
The liquid may be an irritant to theeyes. The teratomeric properties of this com pound were observed in rats and mice. Thesymptoms were embryo deaths and develop mental abnormalities in the urogenital andmusculoskeletal systems.
No carcinogenic actions in animals orhumans have been reported. The histidinereversion–Ames test for mutagenicity wasinconclusive.
Fire Hazard
2,2-DICHLORO-1,1-DIFLUOROETHYL METHYL ETHER is combustible.
Clinical Use
Methoxyflurane is seldom used because of its propensity to cause renal toxicity. It is the most potent agent, and it has the highest solubility in blood. Induction and recovery would be expected to be slow. Chemically, it is rather unstable, and as much as 50% of an administered dose can be metabolized. Toxic metabolites significantly limit its utility as a general anesthetic.
methoxyflurane Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Balaji Enterprise | 08048250356Ext 868 | Mumbai, India | 1 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23556 | 58 | Inquiry |
| CD Chemical Group Limited | +8615986615575 | China | 20356 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| SUZHOU SENFEIDA CHEMICAL CO.,LTD | +86-0512-83500002 +8615195660023 | China | 23053 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | China | 9311 | 58 | Inquiry |
76-38-0(methoxyflurane)Related Search:
1of4
chevron_right




