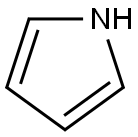
Pyrrole
- CAS No.
- 109-97-7
- Chemical Name:
- Pyrrole
- Synonyms
- 1H-PYRROLE;AZOLE;Pyrroline;Pyrrol;Parzate;IMIDOLE;1H-Pyrrole 98%;PYRROLE;Pyrrhol;FEMA 3386
- CBNumber:
- CB3852794
- Molecular Formula:
- C4H5N
- Molecular Weight:
- 67.09
- MOL File:
- 109-97-7.mol
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | -23 °C (lit.) |
|---|---|
| Boiling point | 131 °C (lit.) |
| Density | 0.967 g/mL at 25 °C (lit.) |
| vapor density | 2.31 (vs air) |
| vapor pressure | 8.7 hPa (20 °C) |
| refractive index |
n |
| FEMA | 3386 | PYRROLE |
| Flash point | 92 °F |
| storage temp. | Store at +2°C to +8°C. |
| solubility | 60g/l |
| pka | 15(at 25℃) |
| form | Liquid |
| color | Clear almost colorless to brownish |
| PH | >6 (10g/l, H2O, 20℃) |
| Odor | at 0.10 % in propylene glycol. sweet warm nutty ethereal |
| Odor Type | nutty |
| biological source | synthetic |
| explosive limit | 3.10-14.8%(V) |
| Water Solubility | 60 g/L (20 ºC) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,8014 |
| JECFA Number | 1314 |
| BRN | 1159 |
| Dielectric constant | 7.5(17℃) |
| Stability | Stable. Incompatible with strong acids, strong oxidizing agents. Combustible. |
| InChIKey | KAESVJOAVNADME-UHFFFAOYSA-N |
| LogP | 0.85 |
| CAS DataBase Reference | 109-97-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyrrole(109-97-7) |
| EPA Substance Registry System | 1H-Pyrrole (109-97-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H301-H318-H332 | |||||||||
| Precautionary statements | P210-P233-P280-P301+P310-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 10-20-25-41 | |||||||||
| Safety Statements | 26-37/39-45-39-24-16 | |||||||||
| RIDADR | UN 1992 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | UX9275000 | |||||||||
| F | 8-10-23 | |||||||||
| Autoignition Temperature | 550 °C | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29339900 | |||||||||
| Hazardous Substances Data | 109-97-7(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 137 mg/kg | |||||||||
| NFPA 704 |
|
Pyrrole price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W338605 | Pyrrole ≥98%, FCC, FG | 109-97-7 | 1SAMPLE | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W338605 | Pyrrole ≥98%, FCC, FG | 109-97-7 | 100G | ₹5228.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W338605 | Pyrrole ≥98%, FCC, FG | 109-97-7 | 1KG | ₹26748.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W338605 | Pyrrole ≥98%, FCC, FG | 109-97-7 | 5KG | ₹108498.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.07492 | Pyrrole for synthesis | 109-97-7 | 10ML | ₹2030.01 | 2022-06-14 | Buy |
Pyrrole Chemical Properties,Uses,Production
Chemical Properties
Six π-electrons are distributed over the five ring atoms of pyrrole. Delocalization of these electrons stabilizes the ring and the lone pair of electrons on the nitrogen atom, which is responsible for the usual basicity of nitrogen compounds, is involved in the electron cloud, and is not available for sharing. Hence, pyrrole is an extremely weak base and the pyrrolic nitrogen is not readily susceptible to electrophilic enzymic attack (Damani, 1985). There is a high electron density, however, at all positions of the ring, which causes pyrrole to be reactive toward electrophilic substitution. In general, electrophilic substitution reactions on the neutral molecule occur preferentially at the C-2 or C-5 positions (Jones and Bean, 1977; Damani and Crooks, 1982).
Physical properties
Pyrrole is a colorless to brown liquid that has a sweet, warm-ethereal smell, similar to chloroform. It dissolves in ethanol, ether, benzene, dilute acids, and most non-volatile oils but does not dissolve in water or dilute alkalis. When stored for extended periods, it tends to aggregate and become brown due to the influence of light.
Preparation
By fractional distillation of bone oil (bone oil is obtained by destructive distillation of animal bone) and subsequent purification via the corresponding potassium salt; by thermal decomposition of ammonium mucate in glycerol or mineral oil.
Production Methods
Pyrrole originally was prepared industrially by fractional distillation of coal tar, bone oil or other protein material, and purified through formation of its potassium derivative (Runge, 1834; Michelman, 1925). Later it was produced by heating ammonium mucate with glycerol or mineral oil (Blicke and Powers, 1927; McElvain and Bollinger, 1941). It is now manufactured by addition of ammonia to either acetylene or butadiene. Good yields of pyrrole also may be obtained from the reaction of ammonia with the corresponding heterocyclic compound (furan) in a vapor-phase process at 480° to 500°C, using alumina as a catalyst (Thompson, 1972) or by catalytic reaction of furan with ammonia over a molybdenum or vanadium oxide catalyst at 350-400°C (Bishop and Denton, 1950).
Definition
ChEBI: 1H-pyrrole is a tautomer of pyrrole that has the double bonds at positions 2 and 4. It is a pyrrole and a secondary amine. It is a tautomer of a 2H-pyrrole and a 3H-pyrrole.
General Description
Pyrrole is one of the flavor compounds that is formed in thermally processed foods due to the Maillard reaction.
Hazard
Moderate fire risk. Toxic by ingestion and inhalation.
Health Hazard
Pyrrole is harmful if swallowed, inhaled, or absorbed through the skin. Its vapor or mist is irritating to the eyes, mucous membranes and upper respiratory tract (Lenga, 1985; Sax, 1984). Although no cases of occupational disease due to pyrrole have been reported, it has a depressant action on the central nervous system and, in severe intoxication, it is injurious to the liver. Tests indicate that it has moderate cumulative toxicity (Parmegianni, 1983).
Fire Hazard
Combustible liquid; flash point (closed cup) 39°C (102°F); vapor forms explosive mixtures with air; LEL and UEL values are not available. Heating with strong oxidizers can be violent.
Industrial uses
Pyrrole is used to a limited extent as a solvent for polymeric esters, but its primary value lies in its function as a chemical intermediate. It is used in the synthesis of non-heterocyclic compounds (Kozikowski, 1984) and its derivatives have been used in the manufacture of dyes, herbicides, perfumes, and as cross-linking agents for curing resins (Thompson, 1972). Derivatives of pyrrole are utilized in pharmaceutical applications, particularly as anti-inflammation drugs and drugs with central nervous system activity, including antihypertensive effects (Sundberg, 1984); and as antimicrobial agents (Freeman, 1975), such as fungicides (Zirngibl, 1983) and bactericides (Bailey and Johnson, 1973; Bailey et al 1973; Sundberg, 1984). Polymers of pyrrole have been used in the preparation of photoconductive materials. The main utility of poly(pyrrole) has been for the modification of electrode surfaces, although numerous other applications can be envisioned (Heilmann and Rasmussen, 1984).
Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. Flammable liquid when exposed to heat or flame; can react with oxilzing materials. To fight fire, use foam, CO2, dry chemical. Violent reaction with 2-nitrobenzaldehyde. When heated to decomposition it emits highly toxic fumes of NOx.
Purification Methods
Dry pyrrole with NaOH, CaH2 or CaSO4. Fractionally distil it under reduced pressure from CaH2. Store it under nitrogen as it turns brown in air. Redistil it immediately before use. The picrate forms orange-red crystals with m 69o(dec). [Beilstein 20 H 4, 20 I 3, 20 II 3, 20 III/IV 61, 20/5 V 3.]
Pyrrole Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3753 | 58 | Inquiry |
| Dr. Silviu Pharmachem Pvt., Ltd. | +91-8390608382 +91-8390608382 | Mumbai, India | 248 | 58 | Inquiry |
| Aether Industries Limited | +91-261-6603130 +91-2616603130 | Gujarat, India | 145 | 58 | Inquiry |
| Avra Synthesis Pvt Ltd | +914027175796 | New Delhi, India | 1603 | 30 | Inquiry |
| Anand Agencies | 91-20-24454597 | Maharashtra, India | 2332 | 58 | Inquiry |
| HiMedia Laboratories | 91-22-61471919 | Maharashtra, India | 1841 | 58 | Inquiry |
| Pluto International | 09820708841 | Mumbai, India | 57 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| JSK Chemicals | 58 |
| Dr. Silviu Pharmachem Pvt., Ltd. | 58 |
| Aether Industries Limited | 58 |
| Avra Synthesis Pvt Ltd | 30 |
| Anand Agencies | 58 |
| HiMedia Laboratories | 58 |
| Pluto International | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
Related articles
- Application of Pyrrole
- Pyrrole is isoelectronic with a cyclopentadienyl anion but is electrically neutral because of the higher nuclear charge on nit....
- Jan 21,2022
- Properties and Reactions of Pyrrole
- Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH.It is a colorless volatile l....
- Nov 14,2019





