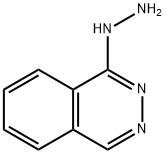
Hydralazine
- CAS No.
- 86-54-4
- Chemical Name:
- Hydralazine
- Synonyms
- c5968;ba5968;c-5068;apressin;ciba5968;apresolin;aprezolin;NSC 126699;apresoline;hidralazin
- CBNumber:
- CB1855362
- Molecular Formula:
- C8H8N4
- Molecular Weight:
- 160.18
- MOL File:
- 86-54-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/4/26 16:55:04
| Melting point | 172°C |
|---|---|
| Boiling point | 276.07°C (rough estimate) |
| Density | 1.2583 (rough estimate) |
| refractive index | 1.5872 (estimate) |
| pka | pKa 6.820± 0.005(H2O,t = 25.0,Iundefined) (Uncertain) |
| Water Solubility | 4.8mg/L(22.5 ºC) |
| BCS Class | 3 |
| CAS DataBase Reference | 86-54-4 |
| IARC | 3 (Vol. 24, Sup 7) 1987 |
| NIST Chemistry Reference | 1(2H)-phthalazinone, hydrazone(86-54-4) |
| EPA Substance Registry System | Hydralazine (86-54-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H301 |
| Precautionary statements | P264-P270-P301+P310+P330-P405-P501 |
| Toxicity | LD50 in mice, rats (mg/kg): 122, 90 orally; 101, 40 i.p. (Dorigotti) |
Hydralazine Chemical Properties,Uses,Production
Description
Cross-reactions between hydrazine derivatives occur. Hydralazine may sometimes cause flushing and reversible Lupus erythematosis
Chemical Properties
Yiellow Solid
Uses
Hydralazine is a non-nucleoside analog that inhibits DNA methylation and reactivates the expression of tumor suppressor genes. Non-selective MAO-A/B inhibitor; semicarbazide-sensitive amine oxidase inhibitor. Antihypertensive.
Definition
ChEBI: The 1-hydrazino derivative of phthalazine; a direct-acting vasodilator that is used as an antihypertensive agent.
Biological Functions
The vasodilation produced by hydralazine (Apresoline) depends in part on the presence of an intact blood vessel endothelium. This implies that hydralazine causes the release of nitric oxide, which acts on the vascular smooth muscle to cause relaxation. In addition, hydralazine may produce vasodilation by activating K+ channels.
Contact allergens
Hydralazine is a hydrazine derivative used as a antihypertensive drug. Skin rashes have been described during treatment. Exposure occurs mainly in the pharmaceutical industry. Cross-sensitivity is frequent with hydrazine, which is considered to be a potent sensitizer.
Mechanism of action
Hydralazine exhibits an antihypertensive effect by directly relaxing smooth muscles of the
vessels. It has an effect on arterial vessels while having a minimal effect on venous vessels.
As a result, resistance of peripheral vessels decreases, and blood pressure is reduced
(diastolic more than systolic).
It does not have a substantial effect on nonvascular smooth musculature or cardiac tissues.
Homeostatic circulatory reflexes remain natural, and the resulting hypotension activates cardiovascular
reflexes, which are expressed as an increase of heart work, power, and volume
of cardiac output. Therefore, it is most effectively used in combination with β-blockers.
Pharmacology
Hydralazine produces widespread but apparently not
uniform vasodilation; that is, vascular resistance is decreased
more in cerebral, coronary, renal, and splanchnic
beds than in skeletal muscle and skin. Renal blood
flow and ultimately glomerular filtration rate may be
slightly increased after acute treatment with hydralazine.
However, after several days of therapy, the
renal blood flow is usually no different from that before
drug use.
In therapeutic doses, hydralazine produces little effect
on nonvascular smooth muscle or on the heart. Its
pharmacological actions are largely confined to vascular
smooth muscle and occur predominantly on the arterial
side of the circulation; venous capacitance is much less
affected. Because cardiovascular reflexes and venous capacitance
are not affected by hydralazine, postural hypotension
is not a clinical concern. Hydralazine treatment
does, however, result in an increase in cardiac
output.This action is brought about by the combined effects
of a reflex increase in sympathetic stimulation of the
heart, an increase in plasma renin, and salt and water retention.
These effects limit the hypotensive usefulness of
hydralazine to such an extent that it is rarely used alone.
Clinical Use
Hydralazine is generally reserved for moderately hypertensive
ambulatory patients whose blood pressure is
not well controlled either by diuretics or by drugs that
interfere with the sympathetic nervous system. It is almost
always administered in combination with a diuretic
(to prevent Na+ retention) and a β-blocker, such
as propranolol (to attenuate the effects of reflex cardiac
stimulation and hyperreninemia). The triple combination
of a diuretic, β-blocker, and hydralazine constitutes
a unique hemodynamic approach to the treatment of hypertension,
since three of the chief determinants of
blood pressure are affected: cardiac output (β-blocker),plasma volume (diuretic), and peripheral vascular resistance
(hydralazine).
Although hydralazine is available for intravenous
administration and has been used in the past for hypertensive
emergencies, it is not generally employed for
this purpose. The onset of action after intravenous injection
is relatively slow, and its actions are somewhat
unpredictable in comparison with those of several other
vasodilators.
Side effects
Most side effects associated with hydralazine administration
are due to vasodilation and the reflex hemodynamic
changes that occur in response to vasodilation.
These side effects include headache, flushing, nasal congestion,
tachycardia, and palpitations. More serious
manifestations include myocardial ischemia and heart
failure. These untoward effects of hydralazine are
greatly attenuated when the drug is administered in
conjunction with a β-blocker.
When administered chronically in high doses, hydralazine
may produce a rheumatoidlike state that
when fully developed, resembles disseminated lupus
erythematosus.
Purification Methods
It crystallises from MeOH. UV: max 656nm at pH ~11. It complexes with Bi3+ , Zn2+ , Fe2+ and Co2+ .
Hydralazine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Cadila Pharmaceuticals Ltd | +91-7069076657 +91-7069076657 | Ahmedabad, India | 54 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Rhyme Organics And Chemicals Limited | 08048372046Ext 929 | Hyderabad, India | 102 | 58 | Inquiry |
| Libertas | 08069033735Ext 211 | Gujarat, India | 29 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21667 | 55 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Xi'an MC Biotech, Co., Ltd. | 029-89275612 +8618991951683 | China | 2255 | 58 | Inquiry |
86-54-4(Hydralazine)Related Search:
1of4
chevron_right




