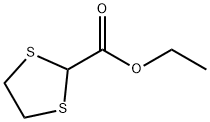
ETHYL 1,3-DITHIOLANE-2-CARBOXYLATE
- CAS No.
- 20461-99-8
- Chemical Name:
- ETHYL 1,3-DITHIOLANE-2-CARBOXYLATE
- Synonyms
- 2-Carboethoxydithiolane;2-Carboethoxy-1,3-dithiolane;2-Ethoxycarbonyl-1,3-dithiolane;Ethyl 1,3-Ditholane-2-carboxylate;ETHYL 1,3-DITHIOLANE-2-CARBOXYLATE;ETHYL GLYOXYLATE ETHYLENE THIOACETAL;Ethyl 1,3-dithiolane-2-carboxylate,99%;Ethyl 1,3-dithiolane-2-carboxylate, 98+%;Ethyl 1,3-dithiolane-2-carboxylate, GC 99%;Ethyl 1,3-dithiolane-2-carboxylate, 99% 5ML
- CBNumber:
- CB2105967
- Molecular Formula:
- C6H10O2S2
- Molecular Weight:
- 178.27
- MOL File:
- 20461-99-8.mol
- Modify Date:
- 2025/1/27 9:38:02
| Boiling point | 85 °C/0.1 mmHg (lit.) |
|---|---|
| Density | 1.249 g/mL at 25 °C (lit.) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| form | Liquid |
| color | Clear light yellow |
| Specific Gravity | 1.249 |
| BRN | 118157 |
| CAS DataBase Reference | 20461-99-8(CAS DataBase Reference) |
ETHYL 1,3-DITHIOLANE-2-CARBOXYLATE Chemical Properties,Uses,Production
Chemical Properties
clear light yellow liquid
General Description
Ethyl 1,3-dithiolane-2-carboxylate is an α-keto acid equivalent. It participates in the conjugate additions to enones. It is a bulky equivalent of acetate undergoing syn-selective aldol reactions. Anion derived from ethyl 1,3-dithiolane-2-carboxylate participates as nucleophile during the total synthesis of the corynanthe alkaloid dihydrocorynantheol and the formal syntheses of the indole alkaloids tacamonine, rhynchophylline and hirsutine. It also participates in the conjugate addition of enolates to phenylglycinol-derived α,β-unsaturated δ-lactams.
Purification Methods
Dissolve the ester in CHCl3, wash it with aqueous K2CO3, twice with H2O, dry it over MgSO4, filter, evaporate and distil the residue in vacuo. [Hermann et al Tetrahedron Lett 2599 1973, Corey & Erickson J Org Chem 36 3553 1971]. [Beilstein 19/7 V 225.]
ETHYL 1,3-DITHIOLANE-2-CARBOXYLATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29808 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | China | 52849 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | China | 18137 | 58 | Inquiry |
| PT CHEM GROUP LIMITED | +86-85511178; +86-85511178; | China | 35425 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| Aladdin Scientific | United States | 52924 | 58 | Inquiry | |
| Amadis Chemical Company Limited | 571-89925085 | China | 131957 | 58 | Inquiry |
20461-99-8(ETHYL 1,3-DITHIOLANE-2-CARBOXYLATE)Related Search:
1of3
chevron_right




