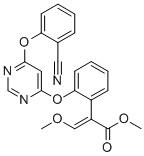
Azoxystrobin
- CAS No.
- 131860-33-8
- Chemical Name:
- Azoxystrobin
- Synonyms
- Azoxystrobin (free acid);QUADRIS;Abound;AMISTAR;HERITAGE;ICI A5504;AZOXYSTROBIN;pyroxystrobin;Azoxystrobin-D3;Azoxystrobin 13C4
- CBNumber:
- CB2189631
- Molecular Formula:
- C22H17N3O5
- Molecular Weight:
- 403.39
- MOL File:
- 131860-33-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:03
| Melting point | 118-119° |
|---|---|
| Boiling point | 581.3±50.0 °C(Predicted) |
| Density | 1.33 |
| vapor pressure | 1.1 x 10-10 Pa (25 °C) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform: Slightly Soluble |
| Colour Index | 23860 |
| Water Solubility | 6 mg l-1 (20 °C) |
| form | Solid |
| pka | -0.93±0.18(Predicted) |
| color | White to yellow |
| InChI | InChI=1S/C22H17N3O5/c1-27-13-17(22(26)28-2)16-8-4-6-10-19(16)30-21-11-20(24-14-25-21)29-18-9-5-3-7-15(18)12-23/h3-11,13-14H,1-2H3/b17-13+ |
| InChIKey | WFDXOXNFNRHQEC-GHRIWEEISA-N |
| SMILES | C1(=CC=CC=C1OC1N=CN=C(OC2=CC=CC=C2C#N)C=1)/C(=C\OC)/C(=O)OC |
| LogP | 2.500 |
| CAS DataBase Reference | 131860-33-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Azoxystrobin(131860-33-8) |
| EPA Substance Registry System | Azoxystrobin (131860-33-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H331-H410 | |||||||||
| Precautionary statements | P261-P271-P273-P304+P340+P311-P391-P403+P233 | |||||||||
| Hazard Codes | T;N,N,T | |||||||||
| Risk Statements | 23-50/53 | |||||||||
| Safety Statements | 22-45-60-61 | |||||||||
| RIDADR | UN 2811 | |||||||||
| WGK Germany | 2 | |||||||||
| Toxicity | LD50 in rats (mg/kg): >5000 orally; >2000 dermally (Godwin) | |||||||||
| NFPA 704 |
|
Azoxystrobin Chemical Properties,Uses,Production
Description
Azoxystrobin is a second broad-spectrum strobilurin fungicide (28,29). It forms white solids with a mp = 116 ?C; vp = 1.1 × 10?7 mPa at 25 ?C; logP = 2.5 at 20?C; water solubility = 6 mg/L; and a half-life of 11 to 17 days for aqueous photolysis.
Chemical Properties
White to beige crystalline solid or powder.
Uses
Azoxystrobin has a very broad spectrum of activity and is active against fungal pathogens from all four taxonomic groups, the Oomycetes, Ascomycetes, Deuteromycetes and Basidiomycetes. It controls diseases on cereals, rice, vines, apples, peaches, bananas, citrus, curcurbits, potatoes, tomatoes, peanuts, coffee and turf.
Definition
ChEBI: An aryloxypyrimidine having a 4,6-diphenoxypyrimidine skeleton in which one of the phenyl rings is cyano-substituted at C-2 and the other carries a 2-methoxy-1-(methoxycarbonyl)vinyl substituent, also at C-2. An inhibitor of mitochondrial respiration by b ocking electron transfer between cytochromes b and c1, it is used widely as a fungicide in agriculture.
Hazard
Moderately toxic by inhalation.
Agricultural Uses
Fungicide: Azoxystrobin has been processed as a Reduced Risk pesticide for Turf uses. Azoxystrobin is a systemic, broad-spectrum fungicide that was first introduced in 1998. It inhibits spore germination and is used on grape vines, cereals, potatoes, apples, bananas, citrus, tomatoes and other crops. Largest crop uses in California are on almonds, rice, pistachios, wine grapes, raisins and garlic. Among the diseases it controls are rusts, downey and powdery mildew, rice blast and apple scab. A U.S. EPA restricted Use Pesticide (RUP)
Trade name
ABOUND®; AMISTAR®; AMISTAR OPTI; AMISTAR PRO; BANKIT®; HERITAGE®; ICIA5504 80WG®; OLYMPUS®; ORTIVA®; PROTEGE®; PROTEGE-ALLEGIANCE WP®;PROTEGE-FL SEED APPLIED FUNGICIDE®; QUADRIS OPTI®; QUILT®; SOYGARD WITH PROTEGE®
Safety Profile
Moderately toxic by inhalation.When heated to decomposition it emits toxic vapors ofNOx.
Potential Exposure
A β-methyoxyacrylate, Strobilurin is an agricultural fungicide.
Metabolic pathway
Azoxystrobin is a recently developed fungicide with a novel mode of
action (see Overview for relevant references). It was first registered in
1996. It has been and is being subjected to the full range of studies to meet
current regulatory requirements for toxicology, metabolism and
environmental fate. Information on its metabolism and environmental
fate was presented as a lecture at the 9th International Congress of
Pesticide Chemistry (IUPAC) in 1998 and this will be published in the
Proceedings. The information presented below is derived from this source
(Joseph, 1998).
Azoxystrobin is a carboxylic acid methyl ester and one of its pathways
of metabolism is via hydrolysis to its carboxylic acid. The latter is not
biologically active (see Overview) and the favourable selective toxicity of
the fungicide is due to this and other metabolic reactions in non-target
species. Azoxystrobin is readily degraded in soil and ultimately mineralised
and it is metabolised in plants and animals. Metabolism is complex
because the molecule possesses several functional groups. Five different
pathways have been identified in plants and animals. Biotransformations
occurring on the parent molecule include hydrolysis, aromatic hydroxylation,
cleavage of both phenoxy linkages, nitrile to amide hydrolysis and
fragmentation of the acrylate double bond. Some photo-induced changes
also occur, including conversion to the (Z)-isomer.
Metabolism
Metabolites are also rapidly degraded in soil. Photolysis studies show a DT50 of 11 days. Due to the rapid degradation and low soil mobility, no leaching is found in field studies.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Combustible; dust may form explosive with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Dispose of contents and container to an approved waste disposal plant. Use a licensed professional waste disposal service to dispose of this material. Ultimate disposal of the chemical, product, and waste containers must consider: the material’s impact on air quality; potential migration in soil or water; effects on animal and plant life; and conformance with public health, local, state, and federal health and environmental regulations. Never reuse or recycle used product containers unless the recycling program specifically accepts pesticide containers and you follow the program’s instructions for preparing the empty containers for collection
Azoxystrobin Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Indofil Industries Limited | +91-2266637373 +91-2266637373 | Maharashtra, India | 16 | 58 | Inquiry |
| Bhagiradha Chemicals And Industries Limited | +91-4042212323 +91-4042221212 | Telangana, India | 12 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| AS WATER CO., LTD. | +86-18994963526 +8618994963526 | United Kingdom | 176 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12460 | 58 | Inquiry |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | China | 213 | 58 | Inquiry |
| Hebei Dangtong Import and export Co LTD | +8615632927689 | China | 984 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2503 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20366 | 58 | Inquiry |
131860-33-8(Azoxystrobin)Related Search:
1of4
chevron_right




