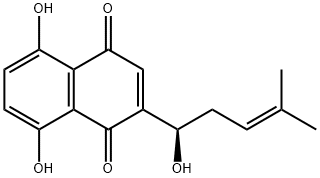
Shikonin
- CAS No.
- 517-89-5
- Chemical Name:
- Shikonin
- Synonyms
- Isoarnebin 4;SHIKONIN;Shikonine;c.i.75535;NSC 252844;SHIKONIN(AS);Tokyo Violet;(-)-SHIKONIN;gromwell red;SHIKONIN 98+%
- CBNumber:
- CB2251044
- Molecular Formula:
- C16H16O5
- Molecular Weight:
- 288.3
- MOL File:
- 517-89-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/23 17:06:52
| Melting point | 148℃ |
|---|---|
| Boiling point | 567.4±50.0 °C(Predicted) |
| Density | 1.373±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO : ≥ 31 mg/mL (107.53 mM); |
| pka | 7.34±0.20(Predicted) |
| form | Red crystalline solid. |
| color | Brown |
| LogP | 4.350 (est) |
| CAS DataBase Reference | 517-89-5(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352-P337+P313-P305+P351+P338-P362+P364-P332+P313 |
| Risk Statements | 20/21/22-50/53-20 |
| Safety Statements | 26-36/37/39-61 |
| RIDADR | 3077 |
| HS Code | 29146990 |
Shikonin Chemical Properties,Uses,Production
Description
Shikonin could be found in the plant of Lithospermum erythrorhizon Sieb. et. Zucc
and Arnebia euchroma (Royle) Johnst. It could also be produced using plant cell
culture techniques. Shikonin is recorded in British Pharmacopoeia as a reference
compound.
Zicao, a traditional Chinese medicine firstly recorded in Shen Nong’s Herbal Classic, has long been used medically in history . Chinese Pharmacopoeia
(Edition 2015) recorded the root of Arnebia euchroma (Royle) Johnst and Arnebia
guttata Bge as Zicao, which is mainly used for inflammatory diseases such as macular
eruptions, measles, sore throat, carbuncles, and burns.
Chemical Properties
Purple-brown crystals with a needle-like shape, melting at 147 ° C, with a rotativity of αD20 = +135 ° (in benzene). It dissolves readily in phenethyl ether, acetone, chloroform, methanol, ethanol, glycerol, animal and vegetable oils, and alkaline aqueous solutions, but is insoluble in water. Its color changes depending on the pH value: at pH 4-6, it turns red; at pH 8, it becomes purple; and at pH 10-12, it appears blue. It exhibits excellent resistance to light, heat, and oxidation, but is reactive to reducing agents, causing the formation of dark purple when exposed to iron ions. Additionally, Shikonin has certain antibacterial effect.
History
Kuroda and Majima firstly identified acetyl shikonin from L. erythrorhizon in 1922 , followed by the discovery of other shikonin derivatives, including shikonin. The chemical structure of shikonin was not precisely identified till 1936 for its high physicochemical similarity with naphthazarin . There have been about 500 publications on shikonin up to now. Great attention has also been paid on the biosynthesis of shikonin and its derivatives, and an increasing number of shikonin derivatives have been designed and synthesized for exploring their antitumor effect. There are two types of derivatives: one is modifications of 1′-OH with parent nucleus naphthazarin remained and the other is modifications of both 1′-OH and parent nucleus naphthazarin, as shown in Fig. 3c, d .
Uses
Shikonin has been used as a red dye for centuries and is reported to possess medicinal properties such as antibacterial, anti-inflammatory and antitumor activities. It occurs as an acetyl derivative in the Japanese shikone, Lithospermum erythrorhizon, another member of the Boraginaceae family. It is the (R)-optical isomer of alkannin. Tissue cultures of L. erythrorhizon are used in Japan to manufacture shikonin mainly for cosmetic use. Both alkannin and shikonin are mordant dyes producing violet to gray colors on fabrics. In Japan, shikonin was used to dye fabrics a color known as Tokyo Violet. Shikalkin the racemate, has been synthesized.
Definition
ChEBI: Shikonin is a hydroxy-1,4-naphthoquinone. It is a naphthoquinone derivative with angiogenesis inhibitor properties.
General Description
Shikonin is a naturally occurring naphthoquinine isolated from the dried root of L. erythrorhizon, an herb used in traditional Chinese medicine. It increases glucose uptake by adipocytes and myocytes and inhibits the activity of phosphatase and tensin homolog (PTEN; IC50 = 2.7 μM). It inhibits glycolysis in cancer cells by inhibiting tumor-specific pyruvate kinase M2 (IC50 = 0.3 μM). Shikonin induces cell death consistent with necroptosis in MCF-7 and HEK293 cancer cell lines. It inhibits leukocyte migration, downregulates chemokine receptor expression, and inhibits HIV-1 replication at nanomolar concentrations. Shikonin exhibits anti-inflammatory activity, reducing joint swelling and cartilage destruction in a mouse model of collagen-induced arthritis.
Pharmacology
Shikonin possesses anti-inflammatory, antioxidant, antiviral, cardiovascular protective,and antitumor activities.
Shikonin reduces inflammation by inhibiting the biosynthesis of leukotrienes and 5-hydroxyeicosatetraenoic acid and thus reduces synthesis of inflammation-related active molecules, which selectively block chemokine binding to CC chemokine receptor 1 .
Shikonin shows free radical scavenging and antioxidant (especially toward superoxide anion and DPPH) activities. It significantly inhibits autoxidation caused by β-carotene and linoleic acid . Its anti-HCV effects have been reported recently with an EC50 at 25 ng/mL, which is lower than that of ribavirin (2.6 μg/mL) .
Recent studies also reveal shikonin possesses cardiovascular protective effects. Shikonin inhibits the activity of TNF-α promoter, revealing its transcriptional antagonism to pro-inflammatory cytokine . Shikonin also shows antitumor potentials by inducing apoptosis and/or necrosis, inhibiting DNA topoisomerase activity and angiogenesis, and regulating proliferative signaling pathways (including MAPK, VEGF, and PTKs ). In addition, shikonin circumvents cancer drug resistance by induction of necroptotic cell death .
Clinical Use
Shikonin and its derivatives have not been approved for clinical use yet. Studies are confined to cellular and animal experiments. Its original plant Zicao has a long history of medical use both orally and externally in China. Various dosage forms of Zicao, including tablets, injections, oils, creams, tinctures, plastics, and pastes, have been developed for different clinical applications especially in dermatology, gynecology, pediatrics, ophthalmology, and otorhinolaryngology. Among them, puccoon oil and lithospermum cream are the most widely used forms.
Shikonin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Wuhan Haorong Biotechnology Co.,ltd | +86-18565342920; +8618565342920 | China | 284 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21677 | 55 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9338 | 55 | Inquiry |
| Chengdu Greenpure Biopharma CO.,Ltd | +8618283602253 | China | 952 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29901 | 58 | Inquiry |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 | CHINA | 1824 | 58 | Inquiry |
| Chengdu Biopurify Phytochemicals Ltd. | +8618080483897 | China | 3424 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19553 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |





