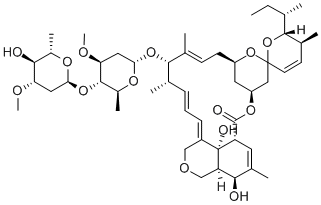
Abamectin
- CAS No.
- 71751-41-2
- Chemical Name:
- Abamectin
- Synonyms
- AVERMECTIN;ABAMECTINE;AVERMECTIN B1;Abamectin 100mg [71751-41-2];Agrimec;DYNAMEC;VAPCOMIC;AVERMECTIN B;Abamectin soL;Abamectin Solution in Acetonitrile
- CBNumber:
- CB2258634
- Molecular Formula:
- C49H74O14
- Molecular Weight:
- 887.11
- MOL File:
- 71751-41-2.mol
- Modify Date:
- 2024/7/24 17:31:20
| Melting point | 150-155°C |
|---|---|
| alpha | D +55.7 ±2° (c = 0.87 in CHCl3) |
| Boiling point | 717.52°C (rough estimate) |
| Density | 1.16 |
| vapor pressure | <2 x 10-7 Pa |
| refractive index | 1.6130 (estimate) |
| Flash point | 150 °C |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO |
| form | Solid |
| Water Solubility | 0.007-0.01 mg l-1 (20 °C) |
| color | White to off-white |
| Merck | 13,2 |
| InChIKey | GVWIWZFXCGTSLL-MSTMYQEVSA-N |
| CAS DataBase Reference | 71751-41-2(CAS DataBase Reference) |
| EPA Substance Registry System | Abamectin (71751-41-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H332-H410 | |||||||||
| Precautionary statements | P261-P264-P270-P273-P301+P310-P304+P340+P312 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 20-28-50 | |||||||||
| Safety Statements | 36/37/39-45-60-61 | |||||||||
| RIDADR | 2588 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CL1203000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 (technical grade) orally in sesame oil in mouse, rat: 13.5, 10.0 mg/kg; dermally in rabbit: >2000 mg/kg; LD50 in mallard duck, bobwhite quail: 84.6, >2000 mg/kg; LC50 (96 hr) in rainbow trout, bluegill: 3.6, 9.6 mg/l; LC50 (48 hr) in Daphnia magna: 0.34 mg/l (Merck Technical Data Sheet) | |||||||||
| NFPA 704 |
|
Abamectin Chemical Properties,Uses,Production
Chemical Properties
Abamectin is a colorless to yellowish crystalline powder. It is soluble in acetone, methanol, toluene, chloroform, and ethanol, but insoluble in water. It is stable, and incompatible with strong oxidizing agents. Abamectin is a mixture of Abamectins containing about 80% Abamectin B1a and 20% Abamectin B1b. These two components, B1a and B1b, have very similar biological and toxicological properties. The Abamectins are insecticidal/miticidal compounds derived from the soil bacterium Streptomyces avermitilis. Abamectin is used to control insect and mite pests of citrus, pear, and nut tree crops, and is used by homeown- ers to control fi re ants. It acts on the nervous system of insects, causing paralyzing effects. Abamectin is a general use pesticide (GUP). It is grouped as toxicity class IV, meaning practically non-toxic, requiring no precautionary statement on its label
Uses
Mixture of Abamectins, containing at least 80% of Abamectin B1a (C48H72O14) and not more than 20% of Abamectin B1b (C47H70O14). Used as acaricide, insecticide
Definition
Any of a group of broad spectrum antiparasitic antibiotics produced by the actinomycete, Streptomyces avermitilis.
Manufacturing Process
1. The contents of a lyophilized tube of Streptomyces avermitilis MA-4680 is
transferred aseptically to a 250 ml Erlenmeyer flask containing 305 ml of
Medium 1: Dextrose 20 g, Peptone 5 g, Meat Extract 5 g, Primary Yeast 3 g,
NaCl 5 g, CaCO3 (after pH adjustment) 3 g, Distilled water 1000 ml, pH 7.0.
The inoculated flask is incubated for 3 days at 28°C on a rotary shaking
machine at a speed of 220 RPM in a 2 inch radius circular orbit. At the end of
this time, a 250 ml Erlenmeyer flask containing 50 ml of Medium 2 [Tomato
Paste 20 g, Modified Starch (CPC) 20 g, Primary Yeast 10 g, CoCl2·6H2O 0.005
g, Distilled water 1000 ml, pH 7.2-7.4] is inoculated with a 2 ml sample from
the first flask. This flask is incubated for 3 days at 28°C on a rotary shaking
machine at a speed of 220 RPM in a 2 inch diameter circular orbit. 50 Ml of
the resulting fermentation broth containing C-076 is effective against an
N.dubius infection in mice.
2. A lyophilized tube of Streptomyces avermitilis MA-4680 is opened
aseptically and the contents suspended in 50 ml of Medium 1 in a 250 ml
Erlenmeyer flask. This flask is shaken for 3 days at 28°C on a rotary shaking
machine 220 RPM with a 2 inch diameter circular orbit. A 0.2 ml portion of
this seed medium is used to inoculate a Slant of Medium 3: Dextrose 10.0 g ,
Bacto Asparagine 0.5 g, K2HPO4 40.5 g, Bacto Agar 15.0 g , Distilled water
1000 ml, pH 7.0. The inoculated slant medium is incubated at 28°C for 10
days and stored at 4°C until used to inoculate 4 more slants of Medium 3.
These slants are incubated in the dark for 8 days. One of these slants is used
to inoculate 3 baffled 250 ml Erlenmeyer flasks containing 50 ml of No. 4
Seed Medium: Soluble Starch 10.0 g, Ardamine 5.0 g, NZ Amine E 5.0 g, Beef
Extract 3.0 g, MgSO4·7H2O 0.5 g, Cerelose 1.0 g, Na2HPO4 0.190 g, KH2PO4
182 g, CaCO3 0.5 g, Distilled water 1000 ml, pH 7.0-7.2. The seed flasks are
shaken for 2 days at 27-28°C on a rotary shaking machine at 220 RPM with a
2 inch diameter circular orbit. The contents of these flasks are pooled and
used to inoculate (5% inoculum) baffled 250 ml Erlenmeyer flasks containing
40 ml of various production media. Flasks containing media 2, 5 and 6 are
incubated for 4 days at 28°C on a rotary shaking machine at 220 RPM with a
2 inch diameter circular orbit. The resulting broth containing C-076 is then
harvested and tested for anthelmintic activity. In all cases 6.2 ml of whole
broth and the solids obtained from centrifuging 25 ml of whole broth are fully
active against N.dubius helminth infections in mice.
3. The one of the four slants of Medium 3 prepared as in Example 2 is used to
inoculate a baffled 250 ml Erlenmeyer flask containing 50 ml of Seed Medium
No. 4. The seed flask is shaken for 1 day at 27- 28°C on a rotary shaking
machine at 220 RPM with a 2 inch diameter circular orbit. The seed flask is
then stored stationary at 4°C until it is ready to be used. The contents of this
flask are then used to inoculate (5% inoculum) 20 unbaffled 250 ml
Erlenmeyer flasks containing 40 ml of Medium No. 2. After 4 days incubation
at 28°C on a rotary shaking machine at 220 RPM with a 2 inch diameter
circular orbit, 19 of the flasks are harvested and pooled. The combined
fermentation broths
containing C-076 are filtered affording 500 ml of filtrate and 84 g of mycelia.
78 G of mycelia are extracted with 150 ml of acetone for ? hour with stirring
and the mixture filtered. The filter cake is washed with 50 ml of acetone and
the filtrate and washings are combined and concentrated to 46.5 ml 30 Ml of
the concentrate is adjusted to pH 4 with dilute hydrochloric acid and extracted
3 times with 30 ml portions of chloroform. The extracts are dried by filtering
through dry Infusorial Earth (Super-Cel) combined and concentrated to
dryness in vacuum. The oily residue of C-076 weighing 91.4 mg is dissolved in
chloroform sufficient to make 3 ml of solution which represents 1% of broth
volume. The C-076 (Abamectin) obtained in this recovery procedure is fully
active against N.dubius infections in mice. In addition, the chloroform
extraction achieved a 70 fold purification of C-076 from the whole broth.
General Description
Odorless off-white to yellow crystals from methanol. Does not hydrolyze in water at pH 3, 5, 7. Used as an acaricide and insecticide.
Reactivity Profile
A lactone.
Hazard
A poison by ingestion. Moderately toxic by inhalation and skin contact.
Health Hazard
Abamectin is an insecticide and miticide. It is very toxic and causes adverse health effects if swallowed and/or inhaled. Emulsifi able concentrate formulations of Abamectin cause slight to moderate eye irritation and mild skin irritation. The symptoms of poisoning observed in laboratory animals include pupil dilation, vomiting, convulsions and/or trem- ors, and coma. Abamectin acts on insects by interfering with the nervous system. At very high doses, laboratory mammals develop symptoms of nervous system depression, inco- ordination, tremors, lethargy, excitation, and pupil dilation. Very high doses have caused death from respiratory failure in animals. Additionally, Abamectin has been reported to cause reproductive effects. Abamectin blocks the nerval conduct system in insects, caus- ing paralysis and death. Laboratory studies have indicated that abamectin may affect the nervous system in experimental animals. A 1-year study with dogs given oral doses of abamectin (0.5 and 1 mg/kg/day) caused adverse health effects, such as pupil dilation, weight loss, lethargy, tremors, and recumbency.
Agricultural Uses
Acaricide, Miticide, Insecticide, Anthelmentic: Used on fruit, vegetable and ornamental crops; pears, citrus fruits, and nut crops; to control mite and insect pests, and also to control household and lawn insects, including fire ants. Approved by the EPA for use in ash trees for control of emerald ash borer. A U.S. EPA restricted Use Pesticide (RUP).
Trade name
ABACIDE®; AFFIRM®; AVID®, AVID-EC®; AVOMEC®; DYNAMEC®; INJECT-A- CIDE AV®; MK 936®(B 1A ); BOVITIN®; DORATECT®; DUOMECTIN®; DUOTIN®; ENDECTO®; ENZEC®; L 676,863® (B 1A ); MK 0936®; MK 936®; PARAFOIL®; VERTIMEC®, VERTIMIL®; VIVID®; ZECTIN®; ZEPHEYR®; ZEPHYR®
Metabolic pathway
Abamectin contains the closely related avermectin B1a and B1b as the
active ingredients. Avermectin B1a contains a sec-butyl moiety whereas
avermectin B1b contains an isopropyl moiety. Chemical degradation and
metabolism studies were conducted with avermectin B1a radiolabelled
with 3H or 14C at various positions of this large molecule. The overall fates
of avermectin B1a and B1b are similar since transformations at the butyl or
propyl moiety were not observed.
Avermectin B1a is stable to hydrolytic degradation, but it is readily
degraded to numerous products in aqueous solutions, soil, glass and
plant foliage/fruit surfaces after light irradiation. Isomerisation and
O-demethylation appear to the primary degradation reactions. In addition,
hydroxylation is a major metabolic reaction in animals. Significant
amounts of the residues in plants and animals were characterised as
unidentified polar components.
Precautions
During use of Abamectin, occupational workers should use safety glasses, gloves, and protective clothing to prevent prolonged skin contact, and work in good ventilation.
Abamectin Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Actis Generics Pvt Ltd | +91-9949094333 +91-9866922779 | AndhraPradesh, India | 44 | 58 | Inquiry |
| Shree Vallabh Chemicals | +91-8849453471 +91-9975490440 | Gujarat, India | 43 | 58 | Inquiry |
| VIVAN Life Sciences Pvt. Limited. | +91(22) 2544 4584 / 2544 4585 | New Delhi, India | 138 | 50 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Susnera Chemicals Pvt. Ltd. | 08048371502Ext 681 | Mumbai, India | 7 | 58 | Inquiry |
| Taj Mahal Vision Chemicals Private Limited | 07942557933 | Mumbai, India | 206 | 58 | Inquiry |
| Orbit Chemicals | 08046069128 | Karnataka, India | 2 | 58 | Inquiry |
| Clearsynth Labs Research Centre | 08048372785Ext 201 | Mallapur, India | 9 | 58 | Inquiry |
Related articles
- The harmful of abamectin to humans
- Avermectin is a new pesticide with a wide margin of safety; however, severe cases of intoxication cause coma, hypotension, ac....
- Feb 19,2024
- Where is Abamectin used
- Abamectin is the key ingredient in insect baits such as Advance 375A, Invict AB Insect Paste,Optiguard Ant Gel, Ascend Fire An....
- Nov 27,2019
71751-41-2(Abamectin)Related Search:
1of4
chevron_right




