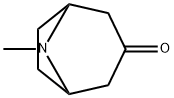
Tropinone
- CAS No.
- 532-24-1
- Chemical Name:
- Tropinone
- Synonyms
- Tropanone;3-Tropinone;8-METHYL-8-AZABICYCLO[3.2.1]OCTAN-3-ONE;Tropanon;Tropinon;ropinone;TROPINONE;NSC 118012;TROPIONONE;3-TROPANONE
- CBNumber:
- CB2470768
- Molecular Formula:
- C8H13NO
- Molecular Weight:
- 139.19
- MOL File:
- 532-24-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/25 20:04:51
| Melting point | 40-44 °C(lit.) |
|---|---|
| Boiling point | 113 °C25 mm Hg(lit.) |
| Density | 1.0268 (rough estimate) |
| refractive index | 1.4598 (estimate) |
| Flash point | 194 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | crystalline |
| pka | 8.93±0.20(Predicted) |
| color | brown |
| PH | 8 (18g/l, H2O, 20℃) |
| BRN | 2329 |
| InChIKey | QQXLDOJGLXJCSE-KNVOCYPGSA-N |
| CAS DataBase Reference | 532-24-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 8-Azabicyclo[3.2.1]octan-3-one, 8-methyl-(532-24-1) |
| EPA Substance Registry System | 8-Azabicyclo[3.2.1]octan-3-one, 8-methyl- (532-24-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302+H332 | |||||||||
| Precautionary statements | P261-P264-P270-P271-P301+P312-P304+P340+P312 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 34-22-36/37/38 | |||||||||
| Safety Statements | 23-24/25-36/37/39-26-22 | |||||||||
| RIDADR | 1544 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29399990 | |||||||||
| NFPA 704 |
|
Tropinone price More Price(10)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T89605 | Tropinone 99% | 532-24-1 | 5G | ₹3301.63 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T89605 | Tropinone 99% | 532-24-1 | 10G | ₹4741.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T89605 | Tropinone 99% | 532-24-1 | 50G | ₹16334.93 | 2022-06-14 | Buy |
| ALFA India | ALF-L02011-22 | Tropinone, 99% | 532-24-1 | 100g | ₹26788 | 2022-05-26 | Buy |
| ALFA India | ALF-L02011-14 | Tropinone, 99% | 532-24-1 | 25g | ₹5381 | 2022-05-26 | Buy |
Tropinone Chemical Properties,Uses,Production
Description
Tropinone (8-methyl-8-azabicyclo[3.2.1]octane-3-one)(7) is the simplest plant tropane and is the first intermediate in the biosynthesis of tropane alkaloids. Studies have called 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoic acid as an intermediate metabolite in tropinone biosynthesis[1]. It is an intermediate of atropine sulfate and is used in the synthesis of atropine. Atropine is an anticholinergic drug. It acts as an M-blocker and is indicated for the relief of visceral colic.
Chemical Properties
Tropinone is a white to light yellow crystal powder, It is an alkaloid and a precursor to a number of additional plant alkaloids. Tropinone is the first intermediate in the biosynthesis of the pharmacologically important tropane alkaloids that possesses the 8-azabicyclo[3.2.1]octane core bicyclic structure that defines this alkaloid class. Chemical synthesis of tropinone was achieved in 1901 but the mechanism of tropinone biosynthesis has remained elusive.
Uses
Tropinone is an oxidative product of Tropane, used to synthesize Morphine and Tropane alkaloids.
Preparation
The first synthesis of tropinone was by Richard Willstätter in 1901. It started from the seemingly related cycloheptanone, but required many steps and had an overall yield of only 0.75%.Willstätter had previously synthesized cocaine from tropinone, in what was the first synthesis and elucidation of the structure of cocaine.
The 1917 synthesis by Robinson is considered a legend in total synthesis due to its simplicity and biomimetic approach. Tropinone is a bicyclic molecule, but the reactants used in its preparation are fairly simple: succinaldehyde, methyl amine and acetone dicarboxylic acid (or even acetone). The synthesis is a good example of a biomimetic reaction or biogenetic-type synthesis because biosynthesis makes use of the same building blocks. It also demonstrates a tandem reaction in a one-pot synthesis. Furthermore the yield of the synthesis was 17% and with subsequent improvements exceeded 90%.
References
1. Arthur J. Birch. Investigating a Scientific Legend: The Tropinone Synthesis of Sir Robert Robinson, F.R.S. Notes and Records of the Royal Society of London, 1993, 47, 277-296. DOI:10.1098/rsnr.1993.0034
2. Andrew J. Humphrey and David O'Hagan. Tropane alkaloid biosynthesis. A century old problem unresolved. Natural Products Reports 2001, 18, 494-502. DOI:10.1039/b001713m
3. Tropinone synthesis via an atypical polyketide synthase and P450-mediated cyclization DOI:10.1038/s41467-018-07671-3
4. R. Robinson (1917). "A synthesis of tropinone". Journal of the Chemical Society, Transaction 111: 762 - 768. doi:10.1039/CT9171100762
Tropinone Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 461 | 58 | Inquiry |
| Orgamine Chemicals(I) Pvt Ltd | +91-9820080281 +91-9820080281 | Maharashtra, India | 451 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt., Ltd. | 91-9324977784 | Maharashtra, India | 160 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Anant Pharmaceuticals Pvt. ltd | +91 8550986868 | Maharashtra, India | 736 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
Related articles
- Tropinone: properties, applications and safety
- Tropinone is a versatile and important compound with diverse applications, but proper use is crucial for safety.
- Nov 20,2023
- The synthesis of Tropinone
- Tropinone is an alkaloid, famously synthesised in 1917 by Robert Robinson as a synthetic precursor to atropine, a scarce commo....
- Aug 24,2021
532-24-1(Tropinone)Related Search:
1of4
chevron_right




