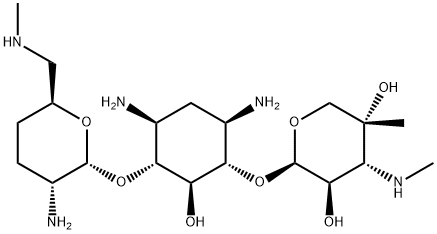
Micronomicin
- CAS No.
- 52093-21-7
- Chemical Name:
- Micronomicin
- Synonyms
- DE 020;KW 1062;XK 62-2;Sagamicin;Santemycin;MICRONOMYCIN;Micronomicin;Gentamicin C2b;Antibiotic XK 62-2;Antibiotic KW 1062
- CBNumber:
- CB31179195
- Molecular Formula:
- C20H41N5O7
- Molecular Weight:
- 463.57
- MOL File:
- 52093-21-7.mol
- Modify Date:
- 2024/11/20 11:41:24
| Melting point | 260° (dec) |
|---|---|
| alpha | D20 +116° (c = 1 in water) |
| Boiling point | 567.39°C (rough estimate) |
| Density | 1.2416 (rough estimate) |
| refractive index | 1.7600 (estimate) |
| storage temp. | 4°C, stored under nitrogen |
| form | Solid |
| pka | 13.29±0.70(Predicted) |
| color | Light yellow to yellow |
| Water Solubility | Water : 250 mg/mL (539.29 mM; Need ultrasonic) |
Micronomicin Chemical Properties,Uses,Production
Description
Micronomicin, formerly called sagamicin, was found in the culture broth of Micromonospora sagamiensis var. nonreducans by Kyowa Hakko Kogyo Company and Abbott Laboratories in 1974. The compound is identical with one of the minor components contained in gentamicin, gentamicin C2b, but it is produced as a single component. Its amino group at the 6 -position is methylated and is not subject to the enzymatic acetylation caused by resistant bacteria, including Pseudomonas aeruginosa. Micronomicin is less toxic than gentamicin to the renal and aural systems.
Uses
Gentamicin C2b (micronomicin) is a trisaccharide pentaaminoglycoside antibiotic belonging to the gentamicin class. Gentamicin C2b was isolated from Micromonospora sagamiensis var. nonreductans by researchers at Kyowa Hakko Kogyo in 1974. Gentamicin C2b has broad activity against Gram positive and Gram negative bacteria. More recently, gentamicin C2b has been shown to induce neuromuscular blockage.
Antimicrobial activity
Antibacterial and pharmacokinetic properties are similar to those of its precursor gentamicin C1a but it is more resistant to AAC(6′). Dosage is similar to that for gentamicin, and should be controlled by blood level determinations. It is available in Japan.
Micronomicin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| BOC Sciences | +1-631-485-4226 | United States | 19552 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 22854 | 58 | Inquiry |
| Shanghai Saikerui Biotechnology Co. , Ltd. | 021-58000709 15900491054 | China | 9522 | 58 | Inquiry |
| Nantong Hanfang Biotechnology Co. , Ltd. | hanfangpharma@126.com; 18616537568 | China | 29981 | 58 | Inquiry |
| Proteintech China | 027-87531629 | CHINA | 6290 | 58 | Inquiry |
52093-21-7(Micronomicin)Related Search:
1of2
chevron_right




