Gentamicin
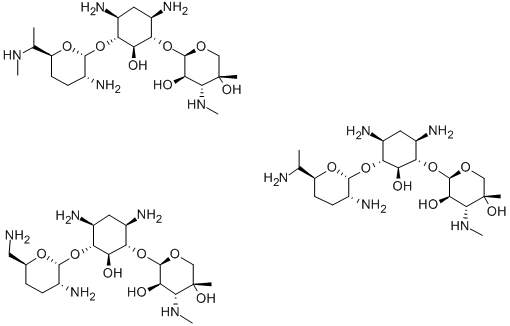
- CAS No.
- 1403-66-3
- Chemical Name:
- Gentamicin
- Synonyms
- GENTAMYCIN;GENTAMYCINE;2-[4,6-Diamino-3-[3-amino-6-(1-methylaminoethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-methylaminooxane-3,5-diol;Gentacycol;GENTAMICINUM;GentamysinsolutionforBiochemistry;Gentavet;Lyramycin;Uromycine;GENTAMICIN
- CBNumber:
- CB7308330
- Molecular Formula:
- C60H123N15O21
- Molecular Weight:
- 1390.71
- MOL File:
- 1403-66-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/23 13:21:54
| Melting point | 102-108° |
|---|---|
| alpha | D25 +146° |
| Density | 1.000 g/cm3 |
| pka | pKa 8.2(66% DMF) (Uncertain);7.9(H2O) (Uncertain) |
| form | powder |
| color | Colorless to light yellow |
| Odor | Odorless |
| CAS DataBase Reference | 1403-66-3(CAS DataBase Reference) |
| EPA Substance Registry System | Gentamicin (1403-66-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H317-H334 |
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Hazardous Substances Data | 1403-66-3(Hazardous Substances Data) |
Gentamicin Chemical Properties,Uses,Production
Description
Gentamicin is a mixture of several antibiotic components produced by fermentation of Mi cromonospora purpurea and other related soil microorganisms (hence its name is spelled with an “i” instead of a “ y”). Gentamicins C-1, C-2, and C-1a are most prominent. Gentamicin is the most important of the aminoglycoside antibiotics still in use. Gentamicin was, for example, one of the first antibiotics to have significant activity against Pseudomonas aeruginosa infections. This water-loving, opportunistic pathogen frequently is encountered in burns, pneumonias, and urinary tract infections.
Chemical Properties
Amorphous solid. Freely soluble in water, pyridine, acid solutions; moderately soluble in methanol, ethanol, and acetone; practically insoluble in benzene and halogenated hydrocarbons.
Uses
antibacterial
Indications
This antibiotic is a combination of three related aminoglycoside
agents obtained from cultures of Micromonospora purpurea and acts
by interfering with the bacterial synthesis of protein. It prevents bacterial protein
synthesis by irreversibly binding to 30S ribosomal subunits. Its antibiotic spectrum
is similar to that of neomycin, and cross-resistance does occur. Gentamicin is active
against gram-negative organisms including Escherichia coli and a high percentage
of strains of Pseudomonas species and other gram-negative bacteria. Proteus
organisms show a variable degree of sensitivity. Some gram-positive organisms,
including S. aureus and group A β-hemolytic streptococci, are also affected.
In
general, higher concentrations are needed to inhibit streptococci than those needed
to inhibit staphylococci and many gram-negative bacteria. It is inactive against
fungi, viruses, and most anaerobic bacteria. The most important use of gentamicin
is in the treatment of systemic gram-negative infections, particularly those due to
Pseudomonas organisms. Widespread use is unwarranted not only because equally
effective drugs are available but also because of the risk of increasing the background
of gentamicin-resistant organisms. Allergic reactions to gentamicin are unusual but
may occur with prolonged use. Cross-reactivity with neomycin may occur.
World Health Organization (WHO)
Gentamicin has been used inin situ preparations for the treatment of minor infections. Antibiotics that are also available for systemic use are not considered acceptable for topical use because of the risk of development of resistance. Neomycin is the topical aminoglycoside listed in the WHO Model List of Essential Drugs.
Antimicrobial activity
It is active against staphylococci, but streptococci
are at least moderately resistant. Gram-positive bacilli, including
Actinomyces and Listeria spp., are moderately susceptible,
but clostridia and other obligate anaerobes are resistant. There
is no clinically useful activity against mycobacteria. It is active
against most enterobacteria, including Citrobacter, Enterobacter,
Proteus, Serratia and Yersinia spp., and against some other aerobic
Gram-negative bacilli including Acinetobacter, Brucella,
Francisella and Legionella spp., although its in-vitro activity
against intracellular parasites such as Brucella spp. is of doubtful
usefulness. It is active against Ps. aeruginosa and other members
of the fluorescens group, but other pseudomonads are
often resistant and Flavobacterium spp. are always resistant.
The MIC for susceptible strains of Ps. aeruginosa can vary
more than 300-fold with the Mg2+ content of the medium.
Activity against Ps. aeruginosa is also significantly lower in serum
or sputum than in ion-depleted broth, as a result both of binding
(more in sputum than in serum) and antagonism by ions.
The action is bactericidal and increases with pH, but to different
degrees against different bacterial species. Marked bactericidal
synergy is commonly demonstrable with β-lactam
antibiotics, notably with ampicillin or benzylpenicillin against
E. faecalis, and with vancomycin against streptococci and
staphylococci. Bactericidal synergy with β-lactam antibiotics
can also be demonstrated in vitro against many Gram-negative
rods, including Ps. aeruginosa. Antagonism with chloramphenicol
occurs in vitro, but this is of doubtful clinical significance.
Like other aminoglycosides, gentamicin is degraded in the
presence of high concentrations of some β-lactam agents.
Acquired resistance
Resistant strains of staphylococci, enterobacteria, Pseudomonas
and Acinetobacter spp. have been reported from many centers,
often from burns and intensive care units where the agent has been used extensively. Overall prevalence rates of resistance
in various countries range from 3% to around 50% for Gramnegative
organisms. Countries in which control of the prescription
of antibiotics is lax often have very high rates.
Acquired resistance in Gram-negative organisms is usually
caused by aminoglycoside-modifying enzymes. The prevalence
of the different enzymes varies geographically. ANT(2″)
is most common in the USA, but in Europe various forms
of AAC(3), particularly AAC(3)-II, are common. ANT(2″)
is also common in the Far East, usually accompanied by
AAC(6′). Strains that owe their resistance to a non-specific
decrease in uptake of aminoglycosides have been involved
in outbreaks of hospital-acquired infection, and are cross-
resistant to all aminoglycosides.
Resistance in staphylococci and high-level resistance in
enterococci is usually caused by the bifunctional APH(2″)-
AAC(6′) enzyme. Other aminoglycoside-modifying enzymes
do not contribute greatly to gentamicin resistance. Gentamicinresistant
staphylococci began to emerge in the mid-1970s. Rates
of resistance in the UK are around 2.5% in methicillin-sensitive
Staph. aureus, 9% in MRSA and 23–73% in coagulase-negative
staphylococci depending on methicillin susceptibility.
High-level resistance to gentamicin (MIC >2000 mg/L) in
E. faecalis is widespread, accounting for around one-third of
blood culture isolates in some places. Penicillin does not exert
synergistic bactericidal activity against such strains, although the
combination of penicillin with streptomycin may remain active.
High-level gentamicin resistance in E. faecium is much less
common,
but has been reported in the UK, the USA and Asia.
Clinical Use
In severe sepsis of unknown origin, gentamicin has been traditionally
combined with other agents. However, monotherapy
has been shown to be as effective as combination therapy.
In systemic Ps. aeruginosa infections it is advisable to combine
gentamicin with an antipseudomonal penicillin or cephalosporin,
owing to likelihood of gentamicin resistance.
Suspected or documented Gram-negative septicemia, particularly when
shock or hypotension is present
Enterococcal endocarditis (with a penicillin)
Respiratory tract infection caused by Gram-negative bacilli
Urinary tract infection
Bone and soft-tissue infections, including peritonitis, burns complicated
by sepsis and infected surgical and traumatic wounds
Serious staphylococcal infection when other conventional antimicrobial
therapy is inappropriate
Gentamicin drops are used for conjunctival infections and for
infections of the external ear. The drug is also used in orthopedic
surgery in bone cements. In these applications systemic
concentrations achieved are negligible and toxicities are
restricted to local effects.
In the elderly and those with renal impairment the dosage
must be suitably modified.
Safety Profile
Poison by intravenous, intraperitoneal, intramuscular, and subcuta neous routes. Mildly toxic by ingestion. Ex perimental teratogenic and reproductive effects. Mutation data reported. Human systemic effects: change in motor activity, changes in vestibular functions, dlstorted perceptions, eye hemorrhage, hallucinations, hdney changes, motor activity changes, trigeminal nerve sensory changes, vestibular function changes, visual field changes. Af fects the peripheral nervous system by intra venous route. An antibiotic. When heated to decomposition it emits acrid smoke and irritating fumes. See also other gentamycin entries.
Gentamicin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| AKASH PHARMA EXPORTS | +91-9388123451 +91-9846039283 | Kerela, India | 470 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| Golden Streak Laboratories Limited | +91 (40) 2726-0320 | New Delhi, India | 15 | 50 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Advance Bio Science | 07942558032 | Gujarat, India | 5 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 | China | 3883 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | China | 5868 | 58 | Inquiry |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 | China | 884 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 | China | 29730 | 60 | Inquiry |
Related articles
- What is Gentamicin?
- Gentamicin is a mixture of relatively equal amounts of three natural aminoglycosides, gentamicin C1, gentamicin C2, and gentam....
- Mar 11,2022
1403-66-3(Gentamicin)Related Search:
1of4
chevron_right




