Clindamycin
- CAS No.
- 18323-44-9
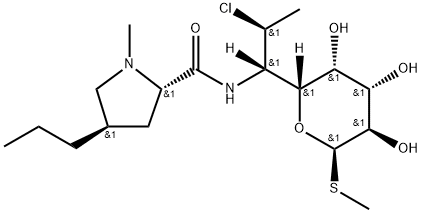
- Chemical Name:
- Clindamycin
- Synonyms
- clindamycine;CLDM;7-cdl;U-21251;sobelin;dalacinc;clinimycin;CLINDAMYCIN;chlolincocin;Clindamycin Base
- CBNumber:
- CB4399999
- Molecular Formula:
- C18H33ClN2O5S
- Molecular Weight:
- 424.98
- MOL File:
- 18323-44-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/14 20:44:35
| alpha | D +214° (chloroform) |
|---|---|
| Boiling point | 134°C (rough estimate) |
| Density | 1.1184 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | Store at -20°C |
| solubility |
DMSO:52.5(Max Conc. mg/mL);123.54(Max Conc. mM) DMF:30.0(Max Conc. mg/mL);70.59(Max Conc. mM) Ethanol:52.5(Max Conc. mg/mL);123.54(Max Conc. mM) PBS (pH 7.2):0.2(Max Conc. mg/mL);0.47(Max Conc. mM) |
| form | A crystalline solid |
| pka | 7.6(at 25℃) |
| color | White to light yellow |
| BCS Class | 1 |
| Stability | Hygroscopic |
| CAS DataBase Reference | 18323-44-9(CAS DataBase Reference) |
| EPA Substance Registry System | L-threo-?-D-galacto-Octopyranoside, methyl 7-chloro-6,7,8-trideoxy-6-[[[(2S,4R)-1-methyl-4-propyl-2-pyrrolidinyl]carbonyl]amino]-1-thio- (18323-44-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H362-H319 | |||||||||
| Precautionary statements | P201-P260-P263-P264-P270-P308+P313-P264-P280-P305+P351+P338-P337+P313P | |||||||||
| Toxicity | LD50 subcutaneous in rat: 2618mg/kg | |||||||||
| NFPA 704 |
|
Clindamycin Chemical Properties,Uses,Production
Description
Clindamycin, a derivative of lincomycin, was first isolated from Streptomyces lincolnesis in 1962 and became commercially available in 1966. The name originates from Lincoln, Nebraska, where the organism was first isolated from a soil sample. The parent compound of clindamycin is lincomycin, which is produced by actinomycete, Streptomyces liconelnensis, which belongs to the order of Actinobacteria. It replaced lincomycin use because of its better absorption and clinical spectrum. Lincomycin belongs to the lincosamides, which is a class of antibiotics. The compounds use as antibiotic agents stems from their ability to interfere with the protein synthesis of bacteria. The chemical modification of lincomycin, clindamycin, has proven to be superior to the natural product for clinical applications. Clindamycin is better absorbed from the gastrointestinal tract and food does not interfere with its absorption. It is also eight times more active against aerobic gram-positive cocci, and it has proven activity against gram-positive and gram-negative anaerobic bacteria as well as protozoal organisms like Toxoplasma and Plasmodium species.
Uses
Clindamycin is a semi-synthetic analogue of lincomycin, prepared by chloride substitution of the exocyclic sugar hydroxy group. This affords a more hydrophobic compound with improved pharmacodynamics. Like other members of the lincosamide family, clindamycin is a broad spectrum antibiotic with activity against anaerobic bacteria and protozoans. Clindamycin acts by binding to the 23S ribosomal subunit, blocking protein synthesis. Clindamycin has been extensively studied with over 8,000 literature citations.
Indications
Clindamycin (Cleocin), 300 to 450 mg/day, is an extremely effective agent for acne.
Definition
ChEBI: Clindamycin is a carbohydrate-containing antibiotic that is the semisynthetic derivative of lincomycin, a natural antibiotic. The physiologic effect of clindamycin is by means of Decreased Sebaceous Gland Activity, and Neuromuscular Blockade.
Contact allergens
This lincosanide antibiotic is used in topical form for acne, or systemically has been responsible for exanthematous rashes and acute generalized exanthematous pustulosis.
Mechanism of action
Clindamycin inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit. The use of clindamycin with macrolides is not recommended since both of them compete for binding sites to the 50S subunit.
Clinical Use
Clindamycin is effective in the treatment of most infections secondary to anaerobes and gram-positive cocci. It can be used for anaerobic pulmonary, intra-abdominal, gynecologic, pelvic, diabetic foot, and decubitus ulcer infections. Another appropriate agent should be added since the majority of these infections are polymicrobial. It can also be used as an alternative agent for patients with severe penicillin allergy. It is also used to treat Clostridium perfringens infection.
Oral preparations of clindamycin and vaginal cream are alternatives to metronidazole for the treatment of bacterial vaginosis. Topical solution is used for treatment of acne vulgaris and rosacea.
Clindamycin is extensively metabolized by the liver and the half-life is prolonged in patients with cirrhosis and hepatitis. Dose reductions are recommended in patients with acute liver disease.
Side effects
The most commonly observed adverse effect is diarrhea. The reported incidence of C. difficile colitis in patients treated with clindamycin varies from 0.1 to 10%. The syndrome may be fatal. If the patient develops C. difficile colitis, clindamycin should be discontinued and the patient should be treated for C. difficile . Other side effects include rash, nausea, vomiting, diarrhea, flatulence, abdominal distension, anorexia, and transient elevation of liver enzymes. Other less common events, such as fever, neutropenia, thrombocytopenia, and eosinophilia have been reported.
Clindamycin Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CHEMXTREE STANDARDS | +919872732255 | Punjab, India | 96 | 58 | Inquiry |
| PROTECH TELELINKS | +91-8571891912 +91-9855060837 | Himachal Pradesh, India | 62 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Jigs Chemical Limited | +91 80 4290 2764 | Ahmedabad, India | 235 | 58 | Inquiry |
| Mahir Technologies Inc. | 09930610606 | Mumbai, India | 38 | 58 | Inquiry |
| Chemland Ind | 08046075772 | Gujarat, India | 95 | 58 | Inquiry |
| Karthik Enterprises | 08048971098 | Mumbai, India | 14 | 58 | Inquiry |
| Taj Mahal Vision Chemicals Private Limited | 07942557933 | Mumbai, India | 206 | 58 | Inquiry |
Related articles
- What is Clindamycin?
- Clindamycin inhibits protein synthesis by binding to the 50S subunit of the ribosome and has a bacteriostatic effect at inhibi....
- Mar 21,2022
18323-44-9(Clindamycin)Related Search:
1of4
chevron_right




