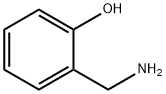
2-Hydroxybenzylamine
- CAS No.
- 932-30-9
- Chemical Name:
- 2-Hydroxybenzylamine
- Synonyms
- 2-(Aminomethyl)phenol;2-HydroxybenzyL;Phenol, 2-(aminomethyl)-;2-HOBA;NSC 127870;Salicylamine;α-AMino-o-cresol;o-HydroxybenzylaMine;2-Hydroxybenzylamine;2-Hydroxybenzylamine,98%
- CBNumber:
- CB4197977
- Molecular Formula:
- C7H9NO
- Molecular Weight:
- 123.15
- MOL File:
- 932-30-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/17 21:30:02
| Melting point | 127 °C |
|---|---|
| Boiling point | 245.0±15.0 °C(Predicted) |
| Density | 1.141±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Chloroform (Slightly), Methanol (Very Slightly, Heated) |
| form | Solid |
| pka | 8.63±0.35(Predicted) |
| color | Beige to Brown |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29222990 | |||||||||
| NFPA 704 |
|
2-Hydroxybenzylamine Chemical Properties,Uses,Production
Description
2-Hydroxybenzylamine (2-HOBA), also known as salicylamide, is a potent γKA scavenger that scavenges γKAs 980-fold faster than the formation of γKA protein adducts and therefore protects cells from the deleterious effects of γKA adducts. 2-HOBA is currently being developed as a nutritional supplement to help protect against the development of conditions associated with dicarbonyl electrophile formation, such as the cognitive decline observed with Mild Cognitive Impairment or Alzheimer's disease.
Chemical Properties
white to light yellow crystal powder.
Uses
2-Hydroxybenzylamine is a potent γ-ketoaldehyde scavenger that has been shown to protects cardiac sodium channel (NaV1.5) from oxidant-induced inactivation.
Production Methods
A simplified method for extracting 2-hydroxybenzylamine from buckwheat is as follows:
1. Dry the roots, stems, and seeds of buckwheat separately. Grind them into a powder using liquid nitrogen.
2. Mix the powder with deionized water and adjust the pH of the solution to 5.0. Add an enzyme mixture consisting of cellulase and pectinase, with a weight ratio of 2:1 to the powder. Perform enzymatic hydrolysis at a temperature of 25°C for 40 minutes. Filter the mixture after hydrolysis.
3. Mix the filter residue obtained in step 2 with 95% ethanol using a solid-liquid ratio of 1g:10mL. Perform ultrasonic extraction at an ultrasound power density of 100W/cm2, ultrasound frequency of 30kHz, extraction temperature of 20°C, and extraction time of 30 minutes. Repeat the extraction process three times, filter the extracts, and combine them.
4. Concentrate the supernatant obtained in step 3 under reduced pressure to obtain a Chemicalbook extract. Add a 3 wt% sodium hydroxide solution to dissolve the extract. Filter the solution to obtain the filtrate.
5. Adjust the filtrate obtained in step 4 to pH 3 using hydrochloric acid, resulting in an adjusted solution. Pass the adjusted solution through an NDA-150 resin column at a temperature of 20°C and a flow rate of 10 BV/h until saturated adsorption is achieved.
6. After reaching saturated adsorption, elute the filtrate obtained in step 4 with an 8 wt% sodium hydroxide solution at 50°C and a flow rate of 2 BV/h. Collect twice the volume of the column volume. Then, elute the filtrate obtained in step 5 with a 3 wt% sodium hydroxide solution at 50°C and a flow rate of 2 BV/h. Collect once the volume of the column volume. Lastly, elute the filtrate obtained in step 5 with water at 60°C and a flow rate of 3 BV/h. Collect once the volume of the column volume. Combine the three eluents.
7. Concentrate the combined eluents under reduced pressure and recrystallize them using edible alcohol. Separate the crystals to obtain 2-hydroxybenzylamine.
Synthesis Reference(s)
Tetrahedron, 48, p. 4301, 1992 DOI: 10.1016/S0040-4020(01)80441-5
2-Hydroxybenzylamine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Arasan Chemicals Private Limited | +91-2513277708 +91-2513277708 | Maharashtra, India | 37 | 58 | Inquiry |
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1879 | 58 | Inquiry |
| Arasan Chemicals Private Limited | +91 (98) 2001-1049 | New Delhi, India | 96 | 0 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 | China | 5828 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16914 | 58 | Inquiry |
| Accela ChemBio Inc. | +1-858-6993322 | United States | 19343 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29825 | 58 | Inquiry |
932-30-9(2-Hydroxybenzylamine)Related Search:
1of4
chevron_right




