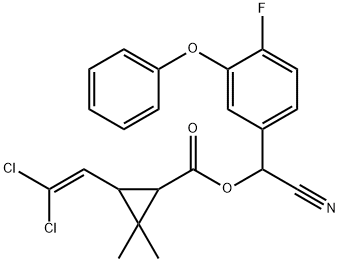
Cyfluthrin
- CAS No.
- 68359-37-5
- Chemical Name:
- Cyfluthrin
- Synonyms
- β-Cyfluthrin;Sofac;BAYGON;SOLFAC;DECATHLON;Baythroid(R);(r,s)-alpha-cyano-4-fluoro-3-phenoxybenzyl-(1r,s)-cis,trans-3-(2,2-dichlorovin;Balecol;tempo 2;bulldock
- CBNumber:
- CB4718934
- Molecular Formula:
- C22H18Cl2FNO3
- Molecular Weight:
- 434.29
- MOL File:
- 68359-37-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/17 18:22:24
| Melting point | 60°C |
|---|---|
| Boiling point | 496.3±45.0 °C(Predicted) |
| Density | 1.3336 (estimate) |
| vapor pressure | 1-8×10-8 Pa (20 °C) |
| refractive index | nD23 1.5511 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform: Slightly Soluble,Methanol: Heated |
| form | Oil |
| Water Solubility | 0.002-0.003 mg l-1 (20 °C) |
| color | Light yellow to yellow |
| Merck | 13,2785 |
| BRN | 2788149 |
| InChIKey | QQODLKZGRKWIFG-UHFFFAOYSA-N |
| LogP | 5.950 |
| NIST Chemistry Reference | Cyfluthrin(68359-37-5) |
| EPA Substance Registry System | Cyfluthrin (68359-37-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300-H331-H410 | |||||||||
| Precautionary statements | P261-P264-P270-P273-P301+P310-P304+P340+P311 | |||||||||
| Hazard Codes | T;N,N,T,T+ | |||||||||
| Risk Statements | 26/28-50/53-28-23 | |||||||||
| Safety Statements | 1/2-36/37/39-45-60-61-28 | |||||||||
| RIDADR | 2588 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GZ1253000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29269090 | |||||||||
| Hazardous Substances Data | 68359-37-5(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in male, female rats, male, female mice (mg/kg): 500-800, 1200, 300, 600 orally in Lutrol (Behrenz); LC50 (96 hr) in rainbow trout: 0.0006 mg/l (Hammann) | |||||||||
| NFPA 704 |
|
Cyfluthrin Chemical Properties,Uses,Production
Description
Cyfluthrin is a pyrethroid insecticide and a modulator of voltage-gated sodium channels (Nav). It slowly activates rat recombinant Nav1.8 channels, delays inactivation by longer than 40 ms, and induces persistent tail currents in channels expressed in X. laevis oocytes. It also decreases the mean burst rate in rat primary neurons (IC50 = 0.99 μM, respectively). Cyfluthrin is toxic to various insects, including A. melinus, G. ashmeadi, E. eremicus, and E. formosa (LC50s = 7, 67, 96, and 63 ng/ml, respectively) and the A. sinensis mosquito (LC50 = 0.446 ppm). It is also toxic to A. mellifera honeybees (LD50 = 0.22 ppm), affecting locomotor activity and wing fanning behavior with an increase in the mean bout duration of time spent upside down, indicating disruption of the righting reflex, and a decrease in wing fanning behavior when administered at a dose of 10 ng/bee. Formulations containing cyfluthrin have been used for the control of insects in agriculture and for non-commercial purposes.
Chemical Properties
In pure form this chemical may be colorless crystalline solid or powder. Commercial is a yellowish paste or viscous, yellowish-brown oil. Aromatic odor.
Uses
Cyfluthrin is used to control a wide range of insects, especially Lepidoptera, in cereals, fruit, vegetables and cotton. It is also used against migratory locusts and grasshoppers, in stored products, in public health situations and in animal health.
Definition
ChEBI: A carboxylic ester obtained by formal condensation between 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid and (4-fluoro-3-phenoxyphenyl)(hydroxy)acetonitrile.
General Description
A viscous amber partly crystalline oil. Used as an insecticide.
Reactivity Profile
Cyfluthrin is a pyrethrine derivative. Incompatible with azocyclotin, and perhaps other azo compounds and organometallics.
Agricultural Uses
Insecticide: Cyfluthrin is a non-systemic insecticide used to control a variety of chewing and sucking insects on cotton, hops, cereals, corn, peanuts, fruit, potatoes and other crops and vegetables. It is also used o control structural pests such as termites.
Trade name
AZTEC®; ATTATOX®; BAY FCR 1272®; BAYTHROID®; BAYTHROID® H; BAYTHROID® TECHNICAL; BUG-B-GON®; CONTUR®; CYLATHRIN®; EULAN SP®; FCR 1272®; INTUDER®; INTUDER HPX®; LASER®; RENOUNCE®; RESPONSAR®; SOLFAC®; TEMPO®; TEMPO® H; TEMPO® 20WP
Potential Exposure
Cyfluthrin is a synthetic pyrethroid, nonsystemic insecticide used to control a variety of chew- ing and sucking insects on cotton, hops, cereals, corn, pea- nuts, fruit, potatoes, and other crops and vegetables. It is also used to control structural pests such as termites. Cyfluthrin can be found in both Restricted Use Pesticides (RUP) and General Use Pesticides (GUP) category. It is also a nitrile.
Metabolic pathway
The non-enzymatic hydrolyzed products of 14C- cyfluthrin are isolated and identified by the incubation reaction mixture of a buffer solution. The products are derived from the hydrolysis of the ester linkage of cyfluthrin via cyanhydrin. This results in the corresponding aldehyde which undergoes both oxidation and reduction to give rise to carboxylic acid and benzyl alcohol, respectively.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3349 (pyrethroid pesticide, solid, toxic)/151 Pyrethroid pesticide, solid toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material UN3439 Nitriles, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
May react violently with strong oxidi- zers, bromine, 90% hydrogen peroxide, phosphorus trichloride, silver powders, or dust. Incompatible with silver compounds. Mixture with some silver compounds forms explosive salts of silver oxalate. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give car- boxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids .
Waste Disposal
Incineration would be an effective disposal procedure where permitted. If an efficient incinerator is not available, the product should be mixed with large amounts of combustible material and contact with the smoke should be avoided. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers.
Cyfluthrin Preparation Products And Raw materials
Raw materials
1of5
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Kilpest India limited | +91-7552586536 +91-7552586536 | Madhya Pradesh, India | 38 | 58 | Inquiry |
| Bayer Vapi Private Limited | +91-2602407123 +91-2602407123 | Gujarat, India | 17 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Mullapudi Enterprises | 08046046233 | Hyderabad, India | 2 | 58 | Inquiry |
| Amarjit Singh & Company | 08046030968 | Haryana, India | 1 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 | China | 5868 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | China | 18137 | 58 | Inquiry |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 | China | 7245 | 58 | Inquiry |





