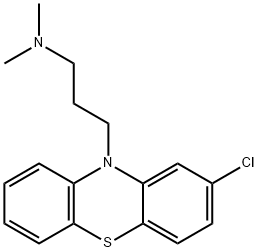
Chlorpromazine
- CAS No.
- 50-53-3
- Chemical Name:
- Chlorpromazine
- Synonyms
- Clorpromazina;Chloropromazine;Thorazine;Chlorpromazin;Prozin;Prazil;2601-A;Esmind;Elmarin;Hibanil
- CBNumber:
- CB5547531
- Molecular Formula:
- C17H19ClN2S
- Molecular Weight:
- 318.86
- MOL File:
- 50-53-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/12/18 14:15:32
| Melting point | 56.5°C |
|---|---|
| Boiling point | bp0.8 200-205° |
| Density | 1.1644 (rough estimate) |
| refractive index | 1.6230 (estimate) |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa 9.3(H2O,t =24±1) (Uncertain) |
| color | White to Off-White |
| NIST Chemistry Reference | Chlorpromazine(50-53-3) |
| EPA Substance Registry System | Chlorpromazine (50-53-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazardous Substances Data | 50-53-3(Hazardous Substances Data) |
| Toxicity | LD50 oral in rat: 142mg/kg |
Chlorpromazine Chemical Properties,Uses,Production
Description
This phenothiazine with sedative properties is used in human medicines and has induced contact dermatitis in nurses or in those working in the pharmaceutical industry. It is also used in veterinary medicine to avoid mortality of pigs during transportation. It is a sensitizer and a photosensitizer.
Chemical Properties
Oily liquid; amine odor.
Uses
In psychiatric practice, chlorpromazine is used in various conditions of psychomotor excitement in patients with schizophrenia, chronic paranoid and also manic-depressive conditions, neurosis, alcohol psychosis and neurosis accompanied by excitement, fear, stress, and insomnia. In comparison with other neuroleptics, chlorpromazine is unique in that it has an expressed sedative effect. It is sometimes used in anesthesiological practice for potentiating narcosis. It also has moderate anticonvulsant action.
Definition
ChEBI: A substituted phenothiazine in which the ring nitrogen at position 10 is attached to C-3 of an N,N-dimethylpropanamine moiety.
Hazard
Toxic by ingestion.
Contact allergens
This phenothiazine with sedative properties is used in human medicine and induced contact dermatitis in nurses or those working in the pharmaceutical industry. It is also used in veterinary medicine to avoid mortality of pigs during transportation. It is a sensitizer and a photosensitizer.
Environmental Fate
Acute and chronic toxicity due to chlorpromazine generally
manifests as an extension of normal pharmacological activity.
The precise mechanism of action of chlorpromazine, and other
phenothiazines, is unknown; however, it is thought to primarily
involve antagonism of dopaminergic (D2) neurotransmission
at synaptic sites and blockade of postsynaptic dopamine
receptor sites at the subcortical levels of the reticular formation,
limbic system, and hypothalamus. This activity contributes to
chlorpromazine’s extrapyramidal reactions. Chlorpromazine
also has strong central and peripheral activity directed against
adrenergic receptors and weak activity against serotonergic,
histaminic (H1), and muscarinic receptors. Chlorpromazine
has slight ganglionic blocking action. Chlorpromazine is
known to depress vasomotor reflexes medicated by the hypothalamus
and/or brain stem; inhibit release of growth hormone;
antagonize secretion of prolactin release-inhibiting hormone;
and reduce secretion of corticotropin-regulatory hormone.
Chlorpromazine also has direct effects on cardiac myocytes;
it can induce early after-depolarizations, block depolarizing
sodium channels, and cause significant prolongation of the
QTc interval.
Chlorpromazine may be irritating to eyes, mucous
membranes, and skin. Contact and inhalation should be
avoided.
Metabolic pathway
The in vivo photodegradation of chlorpromazine in rat skin exposed to UV-A results in the formation of promazine and 2-hydroxypromazine in irradiated rats, but not in the skin of rats kept in the dark. Chlorpromazine sulfoxide is a major metabolite of chlorpromazine, found in smaller quantity in the skin of irradiated rats compared with those kept in the dark. Chlorpromazine sulfoxide is not a photoproduct of chlorpromazine under the experimental conditions.
Chlorpromazine Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Sibram Pharmaceutical | +91-8655777550 +91-8655777550 | Maharashtra, India | 244 | 58 | Inquiry |
| Amoli Organics Pvt Ltd | +91-2266214715 +91-9987040659 | Maharashtra, India | 36 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| Refsyn Biosciences Pvt., Ltd. | 91-413-2200273 | Tamil Nadu, India | 226 | 58 | Inquiry |
| Xiamen Wonderful Bio Technology Co., Ltd. | +8613043004613 | China | 283 | 58 | Inquiry |
| Hangzhou ICH Biofarm Co., Ltd | +86-0571-28186870; +undefined8613073685410 | China | 1015 | 58 | Inquiry |
Related articles
- Uses of Chlorpromazine
- Chlorpromazine is a first-generation (‘typical’) antipsychotic approved by the US Food and Drug Administration (FDA) in 1957.
- Jan 10,2022
50-53-3(Chlorpromazine)Related Search:
1of4
chevron_right




