Decane
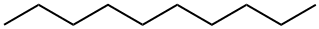
- CAS No.
- 124-18-5
- Chemical Name:
- Decane
- Synonyms
- N-DECANE;Decan;n-Decan;n-Decane, 99.5%;n-Decane for synthesis;DECANE;n-C10H22;N- Decano;ALKANE C10;Decane >
- CBNumber:
- CB5852985
- Molecular Formula:
- C10H22
- Molecular Weight:
- 142.28
- MOL File:
- 124-18-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/30 14:17:11
| Melting point | -30 °C |
|---|---|
| Boiling point | 174 °C(lit.) |
| Density | 0.735 |
| vapor density | 4.9 (vs air) |
| vapor pressure | 1 mm Hg ( 16.5 °C) |
| refractive index |
n |
| Flash point | 115 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.00005g/l |
| form | Liquid |
| color | Colorless |
| Specific Gravity | 0.731 (20/4℃) |
| Relative polarity | -0.3 |
| explosive limit | 0.7-5.4%(V) |
| Odor Threshold | 0.87ppm |
| Water Solubility | INSOLUBLE |
| BRN | 1696981 |
| Henry's Law Constant | 5.59 at 25 °C (calculated from water solubility and vapor pressure, Tolls, 2002) |
| Dielectric constant | 1.8(130℃) |
| Stability | Stable. Incompatible with oxidizing agents. Flammable. |
| InChIKey | DIOQZVSQGTUSAI-UHFFFAOYSA-N |
| LogP | 5.010 |
| CAS DataBase Reference | 124-18-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Decane(124-18-5) |
| EPA Substance Registry System | Decane (124-18-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H226-H304 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P301+P310-P331 | |||||||||
| Hazard Codes | Xn,N,F | |||||||||
| Risk Statements | 10-65-66-67-62-51/53-48/20-38-11 | |||||||||
| Safety Statements | 62-23-16-61-36/37-33-29-9 | |||||||||
| RIDADR | UN 2247 3/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | HD6550000 | |||||||||
| Autoignition Temperature | 410 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29011090 | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| NFPA 704 |
|
Decane price More Price(31)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | D901 | Decane ReagentPlus?, ≥99% | 124-18-5 | 100ML | ₹3575.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D901 | Decane ReagentPlus?, ≥99% | 124-18-5 | 500ML | ₹5754 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.20383 | n-Decane for synthesis | 124-18-5 | 250ML | ₹5360 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.03405 | n-Decane for synthesis | 124-18-5 | 100ML | ₹8040 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.20383 | n-Decane for synthesis | 124-18-5 | 1L | ₹17899.99 | 2022-06-14 | Buy |
Decane Chemical Properties,Uses,Production
Chemical Properties
Decane, C10H22, is a flammable liquid with specific gravity 0.73. Decane is a constituent in the paraffin fraction of crude oil and natural gas. It is released to the environment via the manufacture, use, and disposal of many products associated with the petroleum, gasoline, and plastics industries.
Physical properties
Clear, colorless liquid. Reported odor threshold concentrations were 11.3 mg/m3 by Laffort and Dravnieks (1973) and 620 ppbv by Nagata and Takeuchi (1990).
Uses
Decane is obtained mainly from the refining of petroleum. It is a component of engine fuel and is used in organic synthesis, as a solvent, as a standardized hydrocarbon, and in jet fuel research.
Reactions
The Combustion Reaction of Decane is as follows:
Like all hydrocarbons, decane undergoes hydrocarbon combustion when used as a fuel. The balanced chemical equation for the complete combustion of decane is:
2 C10H22 + 31 O2 → 20 CO2 + 22 H2O + Heat Energy (Enthalpy)
The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The reaction also has a negative enthalpy change (ΔH) value.
General Description
A colorless liquid. Flash point 115°F. Less dense than water and insoluble in water. Vapors heavier than air. In high concentrations its vapors may be narcotic. Used as a solvent and to make other chemicals.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
DECANE is incompatible with oxidizing agents.
Health Hazard
Contact with eyes may produce mild irritation. Contact with skin may cause defatting, redness, scaling, and hair loss. Ingestion may cause diarrhea, slight central nervous system depression, difficulty in breathing and fatigue. Inhalation of high concentrations may cause rapid breathing, fatigue, headache, dizziness, and other CNS effects.
Fire Hazard
Special Hazards of Combustion Products: May produce toxic fumes, including carbon monoxide.
Safety Profile
Questionable carcinogen with experimental tumorigenic data. A simple asphyxiant. Narcotic in high concentrations, Flammable liquid when exposed to heat or flame. Can react with oxidizing materials. Moderately explosive in its vapor form. To fight fire, use foam, CO2, dry chemical. Emitted from modern buildtng materials (CENEAR 69,22,91). See also ARGON for discussion of asphyxiants.
Carcinogenicity
Mice treated with decane developed tumors on the backs, after exposure to ultraviolet radiation at wavelengths longer than 350 nm, generally considered noncarcinogenic. A series of 21 tobacco smoke components and related compounds were applied to mouse skin (50 female ICR/Ha Swiss mice/group) three times weekly with 5 mug/application of benzo[a]pyrene (B[a]P). The test compounds were of five classes: aliphatic hydrocarbons, aromatic hydrocarbons, phenols, and longchain acids and alcohols. Decane was among the compounds that enhanced remarkably the carcinogenicity of B[a]P. and also acted as tumor promoter in two-stage carcinogenesis.
Environmental Fate
Biological. Decane may biodegrade in two ways. The first is the formation of decyl
hydroperoxide, which decomposes to 1-decanol, followed by oxidation to decanoic acid. The other
pathway involves dehydrogenation to 1-decene, which may react with water giving 1-decanol
(Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer and
Finnerty, 1984). The most common degradative pathway involves the oxidation of the terminal
methyl group forming the corresponding alcohol (1-decanol). The alcohol may undergo a series of
dehydrogenation steps, forming decanal, followed by oxidation forming decanoic acid. The fatty
acid may then be metabolized by β-oxidation to form the mineralization products, carbon dioxide
and water (Singer and Finnerty, 1984). Hou (1982) reported 1-decanol and 1,10-decanediol as
degradation products by the microorganism Corynebacterium.
Photolytic. A photooxidation reaction rate constant of 1.16 x 10-11 cm3/molecule?sec was
reported for the reaction of decane with OH in the atmosphere (Atkinson, 1990).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor. Decane
will not hydrolyze because it has no hydrolyzable functional group.
Purification Methods
It can be purified by shaking with conc H2SO4, washing with water, aqueous NaHCO3, and more water, then drying with MgSO4, refluxing with Na and distilling. Also purify through a column of silica gel or alumina. It has been purified by azeotropic distillation with 2-butoxyethanol, the alcohol being washed out of the distillate, using water, the decane is next dried and redistilled. It can be stored with NaH. Further purification can be achieved by preparative gas chromatography on a column packed with 30% SE-30 (General Electric methyl-silicone rubber) on 42/60 Chromosorb P at 150o and 40psig, using helium [Chu J Chem Phys 41 226 1964]. It is soluble in EtOH and Et2O. [Beilstein 1 IV 484.]
Decane Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Vadilal Chemicals Limited | +91-7948936937 +91-7203030735 | Gujarat, India | 39 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| Spectrochem Private Limited | 08048372608Ext 246 | Maharashtra, India | 1056 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| INOX Air Products Pvt. Ltd. | 91-22-41252348 | Maharashtra, India | 53 | 58 | Inquiry |
| Ekta Enterprises | 08046078028 | Uttar Pradesh, India | 10 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Vadilal Chemicals Limited | 58 |
| S D Fine Chem Limited | 58 |
| Spectrochem Private Limited | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| Otto Chemie Pvt. Ltd. | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Central Drug House(P) Ltd. | 58 |
| INOX Air Products Pvt. Ltd. | 58 |
| Ekta Enterprises | 58 |
Related articles
- Toxicological information of decane
- Decane refers to an alkane containing 10 carbon atoms and 22 hydrogen atoms in its molecular structure, the chemical formula i....
- Apr 29,2022
- Toxicity of Decane
- Decane is a constituent in the paraffin fraction of petroleum and is also present in low concentrations as a component of gaso....
- Jan 14,2022
124-18-5(Decane)Related Search:
1of4
chevron_right




