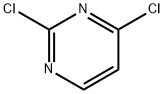
2,4-Dichloropyrimidine
- CAS No.
- 3934-20-1
- Chemical Name:
- 2,4-Dichloropyrimidine
- Synonyms
- 2,4-dichloropyrimidine hydrochloride;2,4DCPY;NSC 20212;NSC 37531;NSC 49119;AKOS 91058;2,4-Dichlorpyrimidin;2,4-Dichloropyrimidi;4-DichloropyriMidine;Pazopanib Impurity 45
- CBNumber:
- CB6202781
- Molecular Formula:
- C4H2Cl2N2
- Molecular Weight:
- 148.98
- MOL File:
- 3934-20-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 57-61 °C (lit.) |
|---|---|
| Boiling point | 101 °C/23 mmHg (lit.) |
| Density | 1.6445 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| Flash point | 101°C/23mm |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | -2.84±0.20(Predicted) |
| form | Powder, Crystals and/or Chunks |
| color | White to yellow to beige or grayish |
| Water Solubility | Soluble in water (partly), methanol, chloroform, and ethyl acetate. |
| Sensitive | Moisture Sensitive |
| BRN | 110911 |
| InChIKey | BTTNYQZNBZNDOR-UHFFFAOYSA-N |
| CAS DataBase Reference | 3934-20-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyrimidine, 2,4-dichloro-(3934-20-1) |
| EPA Substance Registry System | Pyrimidine, 2,4-dichloro- (3934-20-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-28A | |||||||||
| RIDADR | 1759 | |||||||||
| WGK Germany | 3 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29335990 | |||||||||
| NFPA 704 |
|
2,4-Dichloropyrimidine price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 143847 | 2,4-Dichloropyrimidine 98% | 3934-20-1 | 10G | ₹4940 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 143847 | 2,4-Dichloropyrimidine 98% | 3934-20-1 | 50G | ₹13860 | 2022-06-14 | Buy |
| TCI Chemicals (India) | D2310 | 2,4-Dichloropyrimidine min. 98.0 % | 3934-20-1 | 5G | ₹3400 | 2022-05-26 | Buy |
| TCI Chemicals (India) | D2310 | 2,4-Dichloropyrimidine min. 98.0 % | 3934-20-1 | 25G | ₹8200 | 2022-05-26 | Buy |
2,4-Dichloropyrimidine Chemical Properties,Uses,Production
Chemical Properties
White Solid
Uses
2,4-Dichloropyrimidine was used in the synthesis of medicinally important 4-aryl-5-pyrimidinylimidazoles.
Preparation
Obtained by chlorination of uracil. Add uracil, phosphorus trichloride, and xylene amine into the reaction pot, heat to 130 ℃, reflux for about 45min, slightly cool, put in crushed ice, that is, the precipitation of purple solid, filtered while hot and not dissolved, washed with ice water, vacuum drying, a kind of purple crude, decolorized with petroleum ether (boiling range of 60-90 ℃), recrystallized, obtained 2,4-dichloropyrimidine.
General Description
2,4-Dichloropyrimidine is a human skin sensitizer. It undergoes effective one-pot, regioselective double Suzuki coupling reaction to yield diarylated pyrimidines.
Reactivity Profile
The reaction of N-substituted cyclic amines with 2,4-dichloroquinazoline and 2,4-dichloro-5-methyl-pyrimidine afforded 2-amino-4-chloroquinazolines and 2-amino-4-chloro-5-methylpyrimidines, respectively—the reaction of these amines with 2,4-dichloropyrimidine 3a afforded not only 2-amino-4-chloropyrimidines but also the isomeric 4-amino-2-chloropyrimidines. An effective one-pot, regioselective double Suzuki coupling of 2,4-dichloropyrimidine has been developed. This method enables the quick and efficient synthesis of diarylated pyrimidines. The choice of solvent proved critical to the success of this reaction sequence, with alcoholic solvent mixtures affording much greater reactivity and correspondingly lower temperatures than polar aprotic solvents[5-6].
References
[1] DR. AGNES FIZIA. Cyclopalladation in the Periphery of a NHC Ligand as the Crucial Step in the Synthesis of Highly Active Suzuki–Miyaura Cross-Coupling Catalysts[J]. Chemistry - A European Journal, 2017. DOI:10.1002/chem.201702877.
[2] FABIAN BRUENING Lucie E L. Highly Regioselective Organocatalytic SNAr Amination of 2,4-Dichloropyrimidine and Related Heteroaryl Chlorides[J]. European Journal of Organic Chemistry, 2017. DOI:10.1002/ejoc.201700459.
[3] ANDERSONSAMANTHA C HandyScott T. One-pot Double Suzuki Couplings of Dichloropyrimidines.[J]. Synthesis-Stuttgart, 2010. DOI:10.1055/s-0030-1258150.
[4] TOMá? KUBELKA. Synthesis of 2,4-Disubstituted Pyrimidin-5-yl C-2′-Deoxyribonucleosides by Sequential Regioselective Reactions of 2,4-Dichloropyrimidine Nucleosides[J]. European Journal of Organic Chemistry, 2010. DOI:10.1002/ejoc.201000164.
[5] Kenji Yoshida, M. Taguchi. “Reaction of N-substituted cyclic amines with 2,4-dichloroquinazoline, 2,4-dichloropyrimidine, and its 5-methyl derivative.” Journal of The Chemical Society-perkin Transactions 1 102 1 (1992): 919–922.
[6] Samantha C Anderson, Scott T Handy. “One-pot Double Suzuki Couplings of Dichloropyrimidines.” Synthesis-Stuttgart 2010 16 (2010): 2721–2724.
2,4-Dichloropyrimidine Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1262 | 58 | Inquiry |
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| NSR Laboratories Pvt. Ltd. | +91-9866989891 +91-9000457345 | Hyderabad, India | 194 | 58 | Inquiry |
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| LIFECHEM PHARMA | +91-9825440401 +91-9825440401 | Gujarat, India | 25 | 58 | Inquiry |
| HEMANT BIOSCIENCE | 9848655224 | Telangana, India | 40 | 58 | Inquiry |
| SURVIVAL TECHNOLOGIES LIMITED | +91 8108285261 | Gujarat, India | 294 | 58 | Inquiry |
| SRC Laboratories Pvt Ltd | 9676648097 | Hyderabad, India | 21 | 58 | Inquiry |
| Vijayasai Lifesciences | +91 9347036508 | AndhraPradesh, India | 54 | 58 | Inquiry |
| VASISTA LIFE SCIENCES PRIVATE LIMITED | +91-04029554774 +91-9010855544 | Hyderabad, India | 153 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| BTC pharm India | 58 |
| GLR Innovations | 58 |
| NSR Laboratories Pvt. Ltd. | 58 |
| SAGAR LIFE SCIENCES PVT LTD | 58 |
| LIFECHEM PHARMA | 58 |
| HEMANT BIOSCIENCE | 58 |
| SURVIVAL TECHNOLOGIES LIMITED | 58 |
| SRC Laboratories Pvt Ltd | 58 |
| Vijayasai Lifesciences | 58 |
| VASISTA LIFE SCIENCES PRIVATE LIMITED | 58 |
3934-20-1(2,4-Dichloropyrimidine)Related Search:
1of4
chevron_right




