CIS-HEPTACHLOREPOXIDE EXO-, ISOMER B
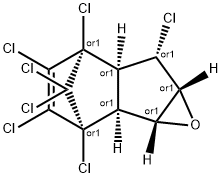
- CAS No.
- 1024-57-3
- Chemical Name:
- CIS-HEPTACHLOREPOXIDE EXO-, ISOMER B
- Synonyms
- HEPTACHLOROEPOXIDE;HCE;HEPTACHLOR-EXO-EPOXIDE;HEPTACHLOR-ENDO-EPOXIDE;beta-Heptachlorepoxide;TRANS-HEPTACHLOR EPOXIDE;TRANS-HEPTACHLOR-ENDO-EPOXIDE;Heptachlor-exo-epoxide (cis-, isomer B);Heptachlor epoxide (Isomer B) 50mg [1024-57-3];CAP1A
- CBNumber:
- CB6250742
- Molecular Formula:
- C10H5Cl7O
- Molecular Weight:
- 389.32
- MOL File:
- 1024-57-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:13
| Melting point | 160-161.5℃ |
|---|---|
| Boiling point | 503.92°C (rough estimate) |
| Density | 1.7335 (rough estimate) |
| vapor pressure | 2.6(x 10-6 mmHg) at 20 °C (IARC, 1974)300(x 10-6 mmHg) at 30 °C (Nash, 1983) |
| refractive index | 1.5000 (estimate) |
| Flash point | 11 °C |
| storage temp. | APPROX 4°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to off-white |
| Water Solubility |
(μg/L): 350 at 25–29 °C (Park and Bruce, 1968) 275 at 25 °C (quoted, Warner et al., 1987) |
| Henry's Law Constant | 0.59(x 10-5 atm?m3/mol) at 5 °C, 0.84 at 15 °C, 1.48 at 20 °C, 2.27 at 25 °C, 3.26 at 35 °C:in 3% NaCl solution: 2.07 at 5 °C, 4.93 at 15 °C, 7.70 at 25 °C, 9.28 at 35 °C (gas stripping-GC, Cetin et al., 2006) |
| Exposure limits | ACGIH TLV: TWA 0.05 mg/m3 (adopted). |
| Stability | Light Sensitive |
| EPA Substance Registry System | Heptachlor epoxide (1024-57-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300-H351-H373-H410 |
| Precautionary statements | P202-P260-P264-P270-P273-P301+P310 |
| Hazard Codes | T,N,F |
| Risk Statements | 25-33-40-50/53-39/23/24/25-23/24/25-11-52/53 |
| Safety Statements | 36/37-45-60-61-16-7 |
| RIDADR | 2761 |
| WGK Germany | 3 |
| RTECS | PB9450000 |
| HazardClass | 6.1(a) |
| PackingGroup | II |
| Toxicity | Acute oral LD50 for rats 47 mg/kg (RTECS, 1985) |
CIS-HEPTACHLOREPOXIDE EXO-, ISOMER B Chemical Properties,Uses,Production
Uses
The cis-metabolite of organochlorine pesticide Heptachlor.
Definition
A degradation product of heptachlor that also acts as an insecticide.
General Description
Heptachlor epoxide is also a white powder. Bacteria and animals break down heptachlor to form heptachlor epoxide. The epoxide is more likely to be found in the environment than heptachlor. Heptachlor epoxide is a degradation product of heptachlor that occurs in soil and in or on crops when treatments with heptachlor, an insecticide, have been made. It forms readily upon exposing heptachlor to air. The U.S. EPA lists heptachlor epoxide as a possible human carcinogen.
Reactivity Profile
CIS-HEPTACHLOREPOXIDE EXO-, ISOMER B may react with acids, bases, and oxidizing and reducing agents.
Hazard
Possible carcinogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: Toxic.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Poison by ingestion and intravenous routes. Human mutation data reported. When heated to decomposition it emits toxic fumes of Cl-. See also HEITACHLOR
Environmental Fate
Biological. In a model ecosystem containing plankton, Daphnia magna, mosquito larva (Culex pipiens quinquefasciatus), ?sh (Cambusia af?nis), alga (Oedogonium cardiacum) and snail (Physa sp.), heptachlor epoxide degraded to hydroxychlordene epoxide (Lu et al., 1975). Using settled domestic wastewater inoculum, heptachlor epoxide (5 and 10 mg/L) did not degrade after 28 days of incubation at 25°C (Tabak et al., 1981). This is consistent with the ?ndings of Bowman et al. (1965). They observed that under laboratory conditions, heptachlor epoxide did not show any evidence of degradation when incubated in a variety of soils maintained at 45°C for 8 days. The soils used in this experiment included Lakeland sand, Lynchburg loamy sand, Magnolia sandy loam, Magnolia sandy clay loam, Greenville sandy clay and Susquehanna sandy clay (Bowman et al., 1965). When heptachlor epoxide was incubated in a sandy loam soil at 28°C, however, 1hydroxychlordene formed at yields of 2.8, 5.8 and 12.0% after 4, 8 and 12 weeks, respectively (Miles et al., 1971).
Photolytic. Irradiation of heptachlor epoxide by a 450-W high-pressure mercury lamp gave two half-cage isomers, each containing a ketone functional group (Ivie et al., 1972). Benson et al. (1971) reported a degradation yield of 99% when an aceton
Graham et al. (1973) reported that when solid heptachlor epoxide was exposed to July sunshine for 23.2 days, 59.3% degradation was achieved. In powdered form, however, only 5 days were required for complete degradation to occur.
Chemical/Physical. Heptachlor epoxide will hydrolyze via nucleophilic attack at the epoxide moiety forming heptachlor diol which may undergo further hydrolysis forming heptachlor triol and hydrogen chloride (Kollig, 1993).
CIS-HEPTACHLOREPOXIDE EXO-, ISOMER B Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Inventichem | +918790275459 | Telangana, India | 155 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Rasayan Trading Co. | 08048606343 | Ahmedabad, India | 75 | 58 | Inquiry |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | China | 20296 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21669 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29825 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 19892 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8930 | 58 | Inquiry |





