Baloxavir marboxil

- CAS No.
- 1985606-14-1
- Chemical Name:
- Baloxavir marboxil
- Synonyms
- Xofluza;S-033188;(3R)-2-[(11S)-7,8-difluoro-6,11-dihydrobenzo[c][1]benzothiepin-11-yl]-9,12-dioxo-5-oxa-1,2,8-triazatricyclo[8.4.0.03,8]tetradeca-10,13-dien-11-yl]oxymethyl methyl carbonate;CS-2794;Baloxavir ester;S-033188;XOFLUZA;Baloxavirmarboxi;aloxavir marboxil;2'S)-Folinic acid;Baloxavir marboxil
- CBNumber:
- CB63361382
- Molecular Formula:
- C27H23F2N3O7S
- Molecular Weight:
- 571.55
- MOL File:
- 1985606-14-1.mol
- Modify Date:
- 2024/8/2 15:22:07
| Boiling point | 712.8±70.0 °C(Predicted) |
|---|---|
| Density | 1.57±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO:45.0(Max Conc. mg/mL);78.73(Max Conc. mM) |
| form | A crystalline solid |
| pka | -1.46±0.40(Predicted) |
| color | White to yellow |
| InChIKey | HKVHAHZGMLTCDW-BWFGELNCSA-N |
| SMILES | C(O)(=O)OC(OC1=C2N(C=CC1=O)N([C@H]1C3=CC=C(F)C(F)=C3CSC3=CC=CC=C31)[C@]1([H])COCCN1C2=O)C |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
Baloxavir marboxil Chemical Properties,Uses,Production
Uses
Baloxavir Marboxil is a prodrug of S-033447, which is an inhibitor of the cap-dependent endonuclease of influenza A and B viruses.
Preparation
The synthesis of baloxavir marboxil involved the following steps: The coupling of the 1-R and 2 fragments was carried out under dehydration conditions of 1-propanephosphonic anhydride (T3P) and methanesulfonic acid at 70 °C to obtain protected baloxavir 19. Compound 19 was then reacted with 0.6 equivalents of PhBr and K2CO3. Debenzylation was then carried out using LiCl in CH3CONMe2 to give baloxavir acid in 94% yield. In the final step for the preparation of the prodrug, baloxavir acid was reacted with chloromethyl methyl carbonate in dimethylacetamide in 93% yield to form baloxavir marboxyl. 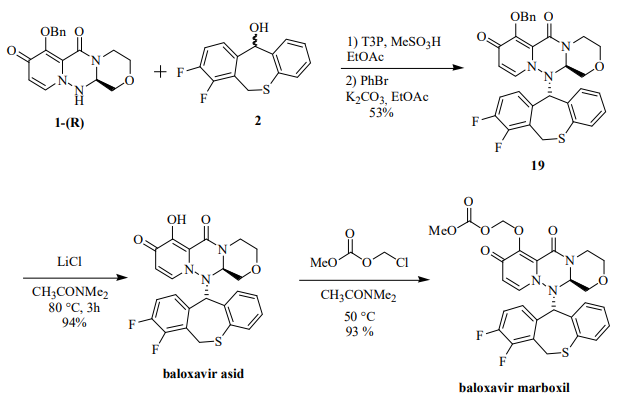
The reaction mechanism for the final step for the synthesis of baloxavir marboxil(BXM).
brand name
XOFLUZA? (baloxavir marboxil)
Biological Activity
Baloxavir marboxil is a prodrug that is metabolised to the active form baloxavir acid also known as S-033447. S-033447 is a small molecule inhibitor of the cap-dependent endonuclease of influenza A and B viruses. It has shown nanomolar antiviral activity against influenza A and B viruses in vitro. In murine models of seasonal influenza and avian influenza A(H5N1) or A(H7N9), orally administered baloxavir showed a rapid reduction in pulmonary viral loads and decreased mortality. Baloxavir significantly reduced the time for alleviation of symptoms and reduced virus titres at 24 and 48 hours post-treatment at three different doses (10 mg, 20 mg and 40 mg) in a phase II study with patients experiencing uncomplicated influenza.
Mechanism of action
Baloxavir marboxil is an influenza therapeutic agent, specifically, an enzyme inhibitor targeting the influenza virus' cap-dependent endonuclease activity, one of the activities of the virus polymerase complex. In particular, it inhibits a process known as cap snatching, by which the virus derives short, capped primers from host cell RNA transcripts, which it then uses for polymerase-catalyzed synthesis of its needed viral mRNAs. A polymerase subunit binds to the host pre-mRNAs at their 5' caps, then the polymerase's endonuclease activity catalyzes its cleavage "after 10–13 nucleotides". As such, its mechanism is distinct from neuraminidase inhibitors such as oseltamivir and zanamivir.
Side effects
Common side effects following the single dose administration of baloxavir marboxil include diarrhea, bronchitis, common cold, headache, and nausea. Adverse events were reported in 21% of people who received baloxavir, 25% of those receiving placebo, and 25% of oseltamivir.
References
[1] HUGHES* D L. Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil[J]. Organic Process Research & Development, 2019, 23 7: 1298-1307. DOI:10.1021/acs.oprd.9b00144.
[2] ANDOYOSHINORI. Pharmacokinetic and pharmacodynamic analysis of baloxavir marboxil, a novel cap-dependent endonuclease inhibitor, in a murine model of influenza virus infection.[J]. Journal of Antimicrobial Chemotherapy, 2021, 76 1: 189-198. DOI:10.1093/jac/dkaa393.
[3] YANGTIANRUI. Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza.[J]. Annals of Pharmacotherapy, 2019, 53 7: 754-759. DOI:10.1177/1060028019826565.
[4] Chemical Structure and Synthesis of Baloxavir Marboxil, A Novel Endonuclease Inhibitor For The Treatment Influenza : An Overview
Baloxavir marboxil Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Siddhivinayakchemicals | +91-912225190111 +91-9819773074 | Mumbai, India | 42 | 58 | Inquiry |
| Bulat Pharmaceutical Pvt Ltd | +91-8448085659 +91-8448085660 | Haryana, India | 123 | 58 | Inquiry |
| Viruj Pharmaceuticals Pvt Ltd | +91-9441591589 +91-9441591589 | Hyderabad, India | 33 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Heading (Nanjing)Pharmtechnologies Co., Ltd. | +86-25-58467899-832 +86-13382406280 | China | 46 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10326 | 58 | Inquiry |
Related articles
- Baloxavir Marboxil: A Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza
- Baloxavir marboxil is a novel influenza treatment with single-dose administration, inhibiting viral gene transcription. Furthe....
- Jan 26,2024
- Introduction to the synthesis method of Baloxavir marboxil
- Baloxavir is a novel cap-dependent endonuclease inhibitor that blocks influenza virus proliferation by inhibiting the initiati....
- Dec 20,2023
- Baloxavir marboxil: mechanism of action, pharmacokinetics and side effects
- Baloxavir marboxil inhibits viral replication in influenza by inhibiting PA activity. It has good tolerability with mild side ....
- Aug 23,2023
Related Qustion
- Q:What is the clinical efficacy of Baloxavir marboxil
- A:A single oral dose of baloxavir marboxil in tablet form improves medication adherence and rapidly reduces viral titres in paed....
- Nov 10,2023





