HYDROGEN SELENIDE
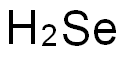
- CAS No.
- 7783-07-5
- Chemical Name:
- HYDROGEN SELENIDE
- Synonyms
- H2Se;[Seh2];selane;Chebi:16503;electronice-2;Electronic E-2;seleniumhydride;Selenwasserstoff;hydrogeneselenie;Selenium hydride
- CBNumber:
- CB6724504
- Molecular Formula:
- H2Se
- Molecular Weight:
- 80.98
- MOL File:
- 7783-07-5.mol
- Modify Date:
- 2024/3/14 15:18:27
| Melting point | -65.73° |
|---|---|
| Boiling point | bp -41.3° |
| Density | d4-42 2.12 |
| solubility | soluble in H2O |
| form | colorless gas |
| color | colorless gas; flammable |
| PH | 3.49(1 mM solution);2.93(10 mM solution);2.41(100 mM solution) |
| Water Solubility | mL/100mL H2O: 377 (4°C), 270 (22.5°C) [MER06]; mL/l00g H2O at standard temp, pressure: 386 (0°C), 351 (10°C), 289 (20°C) [LAN05] |
| Stability | Stable. Highly flammable. Readily forms explosive mixtures with air. Incompatible with oxidizing agents, acids, water, halogenated hydrocarbons. |
| EPA Substance Registry System | Hydrogen selenide (7783-07-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS09,GHS04,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H330-H400-H280-H410 | |||||||||
| Precautionary statements | P273-P391-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P410+P403-P273-P391-P501 | |||||||||
| RIDADR | 2202 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.05 ppm (0.2 mg/m3) | |||||||||
| HazardClass | 2.3 | |||||||||
| IDLA | 1 ppm | |||||||||
| NFPA 704 |
|
HYDROGEN SELENIDE Chemical Properties,Uses,Production
Chemical Properties
colourless gas with a very unpleasant smell
Physical properties
Colorless gas; disagreeable odor; flammable; gas density 3.553 g/L; liquefiesat -41.25°C; liquid density 2.12 g/mL at -42°C; freezes at -65.73°C; criticaltemperature 137.85°C; critical pressure 88.0 atm; soluble in water, carbondisulfide and phosgene; pKa3.89 and 11.0 at 25°C, respectively, for the firstand second replaceable hydrogen.
Uses
Preparation of metallic selenides and organoselenium compounds; in doping as mix for preparation of semiconductor materials containing controlled amounts of significant impurities.
Production Methods
Hydrogen selenide (H2Se) may be formed by the reaction of acids or water with metal selenides or wherever nascent hydrogen is in contact with soluble selenide compounds. It has no commercial use.
Preparation
Hydrogen selenide may be synthesized directly from elements by heatingat elevated temperatures. It is prepared by heating hydrogen at 440°C witheither selenium metal in a sealed tube or with selenium vapor over pumicestone:
H2 + Se → H2Se
Alternatively, the compound may be prepared by treating an alkali metalselenide with hydrochloric acid during mild heating:
NaSe + 2HCl → H2Se + NaCl
Air & Water Reactions
Highly flammable. Soluble in water.
Reactivity Profile
HYDROGEN SELENIDE is a colorless, flammable gas of unpleasant odor, a deadly poison by inhalation. HYDROGEN SELENIDE forms explosive mixtures with air. HYDROGEN SELENIDE reacts vigorously with strong oxidizing reagents, e.g., hydrogen peroxide, nitric acid [Sax, 9th ed., 1996, p.1843].
Hazard
Dangerous fire and explosion risk; reacts violently with oxidizing materials. Toxic by inhalation, strong irritant to skin, damaging to lungs and liver.
Health Hazard
HYDROGEN SELENIDE is a hazardous compound which can cause damage to the lungs and liver. Those experiencing dermatitis, chronic bronchitis, or any form of skin allergy or respiratory tract infection are at a greater risk.
Fire Hazard
HYDROGEN SELENIDE is extremely flammable; may be ignited by heat, sparks, or flames. Vapors may travel to a source of ignition and flash back. Container may explode in heat of fire. HYDROGEN SELENIDE is dangerous and forms explosive mixtures with air. HYDROGEN SELENIDE can decompose into toxic fumes. Incompatible with acid, water, halogenated hydrocarbons, oxidizers, hydrogen peroxide, and nitric acid. HYDROGEN SELENIDE should be stored out of the direct rays of the sun. Keep away from heat and flames.
HYDROGEN SELENIDE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16209 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Central China Special Gas (CCSG) Co., Ltd | 0734-8755555 15674722888 | China | 281 | 58 | Inquiry |
| DWS Specialty Gas Co., Ltd | 159-0619-7626 13194677939 | China | 454 | 58 | Inquiry |
| Portail Substances Chimiques | 10 20 0000 | France | 6027 | 58 | Inquiry |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 | China | 13359 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |





